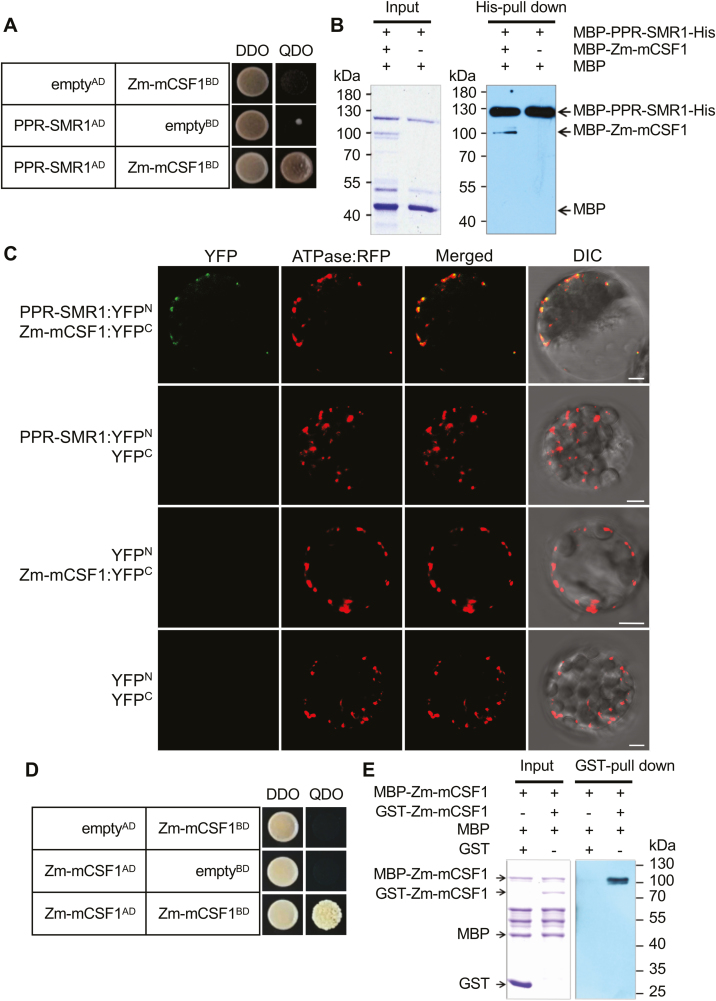
Fig. 5.
PPR-SMR1 protein interacts with Zm-mCSF1. (A) Yeast two-hybrid (Y2H) analysis of PPR-SMR1 and Zm-mCSF1 interaction. The Y2HGold strain harboring the indicated bait and prey constructs were spotted on synthetic dropout (SD) medium without Leu and Trp (double-dropout, DDO) and SD without Ade, Leu, Trp, and His (quadruple-dropout, QDO). Yeast cultures on DDO control plates demonstrate the existence of both plasmids. Positive interactions were verified by growth on QDO plates. (B) Recombinant protein MBP-PPR-SMR1-His interacts with MBP-Zm-mCSF1 as determined using in vitro His pull-down assays. (C) Bimolecular fluorescence complementation (BiFC) analysis of the interaction of PPR-SMR1 and Zm-mCSF1 in mesophyll protoplasts of Arabidopsis. Yellow fluorescent protein (YFP) was split into N- and C-terminus, and PPR-SMR1 was fused with the N-terminus of YFP and Zm-mCSF1 was fused with C-terminus of YFP. Scale bars are 5 µm. (D) Zm-mCSF1AD physically interacts with Zm-mCSF1BD in Y2H assays. Yeast Y2HGold was transformed with paired constructs Zm-mCSF1AD and Zm-mCSF1BD, and AD (activating domain) and BD (binding domain) were used as negative controls. (E) Recombinant protein MBP-Zm-mCSF1 interacts with GST-Zm-mCSF1 as determined using in vitro glutathione S-transferase (GST) pull-down assays. ‘+’ and ‘−’ indicate the presence and absence of the corresponding proteins in the reactions, respectively.