Abstract
Plants have evolved various strategies to sense and respond to saline environments, which severely reduce plant growth and limit agricultural productivity. Alteration to the cell wall is one strategy that helps plants adapt to salt stress. However, the physiological mechanism of how the cell wall components respond to salt stress is not fully understood. Here, we show that expression of XTH30, encoding xyloglucan endotransglucosylase-hydrolase30, is strongly up-regulated in response to salt stress in Arabidopsis. Loss-of-function of XTH30 leads to increased salt tolerance and overexpression of XTH30 results in salt hypersensitivity. XTH30 is located in the plasma membrane and is highly expressed in the root, flower, stem, and etiolated hypocotyl. The NaCl-induced increase in xyloglucan (XyG)-derived oligosaccharide (XLFG) of the wild type is partly blocked in xth30 mutants. Loss-of-function of XTH30 slows down the decrease of crystalline cellulose content and the depolymerization of microtubules caused by salt stress. Moreover, lower Na+ accumulation in shoot and lower H2O2 content are found in xth30 mutants in response to salt stress. Taken together, these results indicate that XTH30 modulates XyG side chains, altered abundance of XLFG, cellulose synthesis, and cortical microtubule stability, and negatively affecting salt tolerance.
Keywords: Arabidopsis, cellulose, microtubule, salt stress, XLFG, XTH30, xyloglucan
Xyloglucan endotransglucosylase-hydrolase30 modulates xyloglucan side chains by altering abundance of xyloglucan-derived oligosaccharide, negatively affecting salt tolerance.
Introduction
Salt stress severely reduces plant growth and limits agricultural productivity (Zhu, 2002, 2016). Plants have evolved various strategies to sense and achieve protection against salt stress (Shi et al., 2000; Munns and Tester, 2008). They sense and transmit the stress signal from cell surface to nucleus, then regulate downstream genes to adaptively respond to salt stress (Julkowska and Testerink, 2015; Zhu, 2016).
The plant cell wall is a structural layer outside the cell membrane that provides the cell with both structural support and protection. One of the important plant adaptations to salt stress is differential regulation of growth, accompanying dynamic changes of the plant cell wall (Tenhaken, 2014; Cosgrove, 2015; Wang et al., 2016). The plant cell wall has a dynamic architecture with cellulose microfibrils embedded in an amorphous matrix of pectin and hemicellulose polysaccharides as well as structural proteins, and in some cells also lignin (Mutwil et al., 2008; Scheller and Ulvskov, 2010). Several genes that are involved in cellulose synthesis, CesA1, CesA6, and CesA8, have been implicated in salt tolerance (Chen et al., 2005; Zhang et al., 2016a). Knocking out either CesA6 or CesA1 confers salt stress sensitivity (Zhang et al., 2016a). Companion of cellulose synthase proteins (CC1 and CC2) interact with CesAs and microtubules, and mutations of CC1 and CC2 led to salt-sensitive phenotypes by altering microtubule and cellulose synthase complex (CSC) behavior (Endler et al., 2015).
Xyloglucan (XyG) is an important hemicellulose polymer of the primary cell wall in dicotyledons and non-commelinid monocotyledons. XyG plays a vital role in loosening or stiffening the cell wall by binding to cellulose microfibrils with hydrogen bonds during cell elongation (Hayashi and Kaida, 2011; Park and Cosgrove, 2015; Pauly and Keegstra, 2016). XyG has a common backbone of (1–4)-linked β-D-glucopyranosyl residues (β-D-Glup), which can be connected with α-D-xylopyranosyl (α-D-Xylp) residues at O-6 (Cosgrove, 2005). A standard nomenclature using a single letter is used to represent XyG connections. G represents unconnected β-D-Glup residue, while X, L, and F indicate Glc residues connected with α-D-Xylp, β-D-Galp-(1–2)-α-D-Xylp, or α-l-Fucp-(1–2)-β-D-Galp-(1–2)-α-D-Xylp side chains, respectively (Fry et al., 1993). XyG chains can be cleaved or rejoined by xyloglucan endotransglucosylase-hydrolase (XTH) (Nishitani and Tominaga, 1992). Many members of the XTH family in plants have been identified including 33 members in Arabidopsis (Yokoyama and Nishitani, 2001), 29 in rice (Yokoyama et al., 2004), and 22 in barley (Strohmeier et al., 2004). It has been shown that XTHs play a vital role in plant development. During cell wall expansion, AtXTH27 is highly expressed and can modulate the generation of tracheary elements in Arabidopsis rosette leaves (Matsui et al., 2005). AtXTH21 plays a vital role in the growth of the primary roots by affecting cellulose deposition (Liu et al., 2007). AtXTH28 is involved in automatic self-pollination (Kurasawa et al., 2009). In addition to regulating developmental growth, XTHs are also involved in the response of plants to abiotic stress. Loss-of-function mutation in AtXTH15 and AtXTH31 enhances aluminum tolerance (Zhu et al., 2012; Shi et al., 2015). Constitutive expression of a Capsicum annuum XTH, CaXTH3, in Arabidopsis or tomato improved salt and drought tolerance (Cho et al., 2006; Choi et al., 2011). Overexpression of Populus euphratica XTH, PeXTH, in tobacco enhanced salt tolerance by the development of leaf succulence (Han et al., 2013). However, the function of XTHs in salt stress and the mechanisms by which XTHs respond to salt stress are still unclear.
In this study, Arabidopsis xyloglucan endotransglucosylase-hydrolase30 (XTH30) was identified as responding to salt stress. XTH30 loss-of-function mutations led to salt tolerance and gain-of-function resulted in salt sensitivity. Moreover, XTH30 modulated XyG structure, cellulose content and depolymerization of microtubules in response to salt stress.
Materials and methods
Plant materials and growth conditions
Arabidopsis (L.) Heynh. ecotype Columbia (Col-0), xth30-1 (CS16544) and xth30-2 (CS16543) mutant were obtained from the Arabidopsis Biological Resource Center (ABRC). Homozygous xth30 mutants were identified by T-DNA insertion-based PCR using the specific primers listed in Supplementary Table S1 at JXB online. The xth30 mutants (xth30-1 and xth30-2) were crossed with transgenic Arabidopsis expressing green fluorescent protein (GFP)-tagged α-tubulin 6 isoform (35S:GFP-TUA6) for the observation of cortical microtubules.
Seeds were sterilized and sown on a solid medium containing half-strength Murashige and Skoog salts including vitamins and 1% (w/v) sucrose at 4 °C for 2 d and then grown in a growth chamber (22 °C, 100–200 µmol m−2 s−1, 14 h light/10 h dark, 60% humidity).
Phenotypic analysis
For a salt sensitivity assay on plates, 5-day-old seedlings grown on 1/2 MS medium were transferred onto 1/2 MS medium with mannitol or salt added as described and allowed to grow for an additional 7 d. Seedlings were photographed and freshly weighed.
For the hydroponic salt sensitivity assay, 3-week-old seedlings were treated with nutrient solution as described in Fang et al. (2016) containing 125 mM NaCl and allowed to grow for an additional 9 d. After recovery with normal nutrient solution for 3 d, the seedlings were photographed and the survival rate was calculated.
For a heavy metal stress (Zn2+ and Cd2+) tolerance test, 5-day-old seedlings grown on 1/2 MS medium were transferred onto 1/2 MS medium with 50 µM CdCl2 and 500 µM ZnSO4, then allowed to grow for an additional 7 d. For ABA treatments, 5-day-old seedlings grown on 1/2 MS medium were transferred onto 1/2 MS medium with 20 µM ABA, then allowed to grow for an additional 7 d. Seedlings were photographed after various treatments.
Determination of Na+ content
Shoots and roots of Arabidopsis seedlings were harvested separately, and Na+ content was measured according to the methods described by Wang et al. (2015). Briefly, dry samples were digested in 1 ml of nitric acid at 90 °C for 12 h, diluted to 5 ml with distilled water, and then analysed using an inductively coupled plasma-optical emission spectrometry instrument (Pekin Elmer, USA).
Hypocotyl length measurements
For isoxaben and oryzalin treatments, seeds were sown on 1/2 MS medium plates supplemented with or without 2 nM isoxaben or 400 nM oryzalin (Sigma-Aldrich, USA) and grown for 6 d in the dark. For salt treatments, 2-day-old etiolated seedlings were transferred to 1/2 MS plates containing 100 mM NaCl for another 5 d in the dark. The hypocotyl lengths were quantified using the ImageJ program.
Vector construction and generation of transgenic plants
The full-length XTH30 with no stop codon was amplified by PCR using the specific primers (Supplementary Table S1) and cloned into the pEarleyGate 101 vector using the BP and LR clonase reaction (Invitrogen, USA). The recombinant plasmid was sequenced and introduced to Col-0 and xth30-1 mutant by Agrobacterium tumefaciens strain GV3101-mediated transformation. Positive transformants were selected on 1/2 MS medium containing 25 µg ml−1 DL-phosphinothricin (Sigma-Aldrich). The resistant T2 seedlings with 3:1 segregation of resistance were transferred to soil to obtain homozygous T3 seeds from individual lines.
Seed germination assays
More than 100 seeds were sown on 1/2 MS medium containing 1% sucrose with or without 125 mM NaCl. The plates were stratified at 4 °C for 48 h, and placed at 22 °C under light conditions. Radicle emergence of >1 mm was counted every day for germination analysis.
Real-time PCR analysis
Total RNA was extracted from seedlings or etiolated hypocotyls using the RNeasy Plant Mini Kit (Qiagen, USA). RNA was first treated with DNase I (Sigma-Aldrich), and first-stand cDNA synthesis was performed using the iScript cDNA Synthesis Kit (Bio-Rad, USA) according to the manufacturer’s protocol. Real-time RT-PCR was performed using SYBR Select Master Mix (Applied Biosystems, USA) on diluted (5 times) cDNA using the StepOne Plus Real-Time PCR System (Applied Biosystem 7500). The specific primers are listed in Supplementary Table S1. The relative expression levels were determined using the 2–ΔΔCT method as described in (Yan et al., 2015).
Determination of H2O2 level
For quantification of H2O2, 3-week-old hydroponically grown seedlings were treated with nutrient solution containing 125 mM NaCl for 6 d, and shoots were harvested. H2O2 content was determined by a horseradish peroxidase-based spectrophotometric assay following the protocol described by Pierce™ Quantitative Peroxide Assay Kit (Aqueous) (Thermo Fisher Scientific, USA).
Antioxidant enzyme assay
Three-week-old hydroponically grown seedlings were treated with nutrient solution containing 125 mM NaCl for 6 d, and shoots were harvested. The samples were homogenized in 1 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpyrrolidone, with the addition of 1 mM sodium ascorbate. The homogenate was centrifuged at 12 000 g for 30 min at 4 °C, and then the supernatant was used for measuring the activities of antioxidant enzymes as previously described (Huang et al., 2013).
Subcellular localization of XTH30
The XTH30 coding sequence was fused to yellow fluorescent protein (YFP) at the N-terminus driven by 35S promoter (XTH30-YFP). Four-week-old Nicotiana benthamiana leaves were co-infiltrated with Agrobacterium tumefaciens strain GV3101 carrying the 35S::XTH30-YFP fusion construct and the AtPIP2A-mCherry marker (pm-rk) as described by Nelson et al. (2007). The plasmolysed tobacco cells were observed by treatment with 300 mM mannitol for 10 min. The microscopy was performed using a Zeiss LSM710 device as previously described (Zhu et al., 2016).
Plasma membrane isolation
The leaves of OX#1 seedlings were harvested and homogenized in a solution (50 mM Tris–HCl, pH 7.5, 1 mM EDTA, 10 mM KCl, 0.5 mM phenylmethylsulfonyl fluoride and 2 mM dithiothreitol) and centrifuged at 6000 g for 10 min at 4 °C. The supernatant was centrifuged at 100 000 g for 45 min, the pellet was used as total microsomal membrane proteins, and the supernatant was regarded as cytosolic proteins.
XTH30 promoter::GUS construct and GUS activity
A 2000 bp promoter region of XTH30 was amplified by PCR using the specific primers listed in Supplementary Table S1. The PCR product was cloned into the pGWB3 vector using the BP and LR clonase reaction (Invitrogen). The recombinant plasmid was sequenced and introduced into Col-0 by Agrobacterium tumefaciens strain GV3101-mediated transformation. Positive transformants were selected on 1/2 MS medium containing 50 μg ml−1 hygromycin (Omega Scientific, USA). Various tissues were submerged in X-Gluc buffer (50 mM sodium phosphate buffer, pH 7.0, 1 mM EDTA, 0.5 mg ml−1 5-bromo-4-chloro-3-indolyl β-D-GlcUA [X-Gluc] (Sigma-Aldrich), 0.4% Triton X-100, 5 mM potassium ferricyanide, and 5 mM potassium ferrocyanide) and incubated at 37 °C overnight in the dark, followed by washing with 70% ethanol to remove chlorophyll as described in Lee et al. (2015).
Cell wall preparation
For quantification of the crystalline cellulose fraction and XyG contents, 2-day-old etiolated seedlings were transferred to 1/2 MS plates containing 100 mM NaCl for another 5 d. The fresh samples were harvested into 96% ethanol and incubated for 30 min at 100 °C to inactivate cell wall-degrading enzymes. The tissue was homogenized using a Retsch MM200 mixer mill and centrifuged. The pellet was washed with 100% ethanol and twice with a mixture of chloroform and methanol (2:1), followed by four successive washes with 100% ethanol and acetone. The pellet was air-dried overnight and is referred to as alcohol-insoluble residue (AIR).
Crystalline cellulose content
The crystalline cellulose content was determined as described (Fang et al., 2016). In brief, the pellet was air-dried overnight. The starch in the samples was degraded with α-amylase, amyloglucosidase, and pullulanase (Megazyme, Brae, Ireland). Destarched AIR (2 mg) was hydrolysed in 2 M trifluoroacetic acid (TFA) at 121 °C for 1 h. Then the TFA-insoluble material was washed with water and further hydrolysed with 72% (v/v) sulfuric acid containing 10 mg myo-inositol for 1 h at room temperature. The sulfuric acid was then diluted to 1 M with water and the samples further incubated at 100 °C for 3 h; they were neutralized with barium carbonate (BaCO3) to provide the crystalline cellulose fraction and analysed by high performance anion exchange chromatography (HPAEC) on an ICS-5000 instrument (Thermo Fisher Scientific) equipped with a CarboPac PA20 (3 mm×150 mm, Thermo Fisher Scientific) analytical anion exchange column, PA20 guard column (3 mm×30 mm), borate trap, and a 500 pulsed amperometric detector. The myo-inositol was used as an internal standard.
MALDI-TOF mass spectrometry analysis of XyG oligosaccharides
To analyse the XyG, we used the rapid phenotyping method using enzymatic oligosaccharide fingerprinting as used in Lerouxel et al. (2002). Two-day-old seedlings grown in the dark on 1/2 MS medium were transferred to 1/2 MS medium with or without 100 mM NaCl, and then grown for 5 d. The hypocotyl was stored in 100% ethanol. After ethanol removal and rehydration, XyG oligosaccharides were generated by treating with 1 unit of xyloglucanase (Megazyme) or endoglucanase (Megazyme) in 50 mM sodium acetate buffer (pH 5.0) overnight at 37 °C. Matrix-assisted laser-desorption ionization time of flight mass spectrometry of the XyG oligosaccharides was recorded with an Applied Biosystems 4800 MALDI/TOF mass spectrometer using super-DHB (Sigma-Aldrich) as matrix.
Xyloglucan content quantified by iodine staining
XyG content was measured using iodine staining as in Kooiman (1960) and Vuttipongchaikij et al. (2012) with minor modifications. In brief, 2-day-old etiolated seedlings were transferred to 1/2 MS plates containing 100 mM NaCl for another 5 d in dark. The AIR was extracted from hypocotyl. Hemicellulose material was isolated from de-starched AIR by incubating with 4 M KOH containing 1% sodium borohydride overnight. The supernatant were neutralized with glacial acetic acid on ice and dialysed in dialysis tubing (Spectra/Por6 3000 MWCO; Spectrum, USA) against water. The dialysed material was freeze-dried and dissolved in water. A 200 μl portion of each sample was mixed with 112 μl of 20% Na2SO4 and 28 μl of Lugol’s solution (Sigma-Aldrich). After incubation for 1 h at room temperature, the absorbance was determined at 640 nm. Tamarind (Tamarindus indica) xyloglucan (Megazyme) was used as a standard.
Microtubule observation
Both wild type and xth30 mutant seeds expressing 35S:GFP-TUA6 were grown on 1/2 MS medium for 3 d and then transferred to 1/2 MS medium with 100 mM NaCl or 10 µM oryzalin. After the indicated treatments, the cortical microtubules in etiolated hypocotyls were observed under a confocal microscope (Zeiss LSM710) with an excitation at 488 nm and emission at 533 nm.
Results
The expression of XTH30 is strongly induced by salt stress
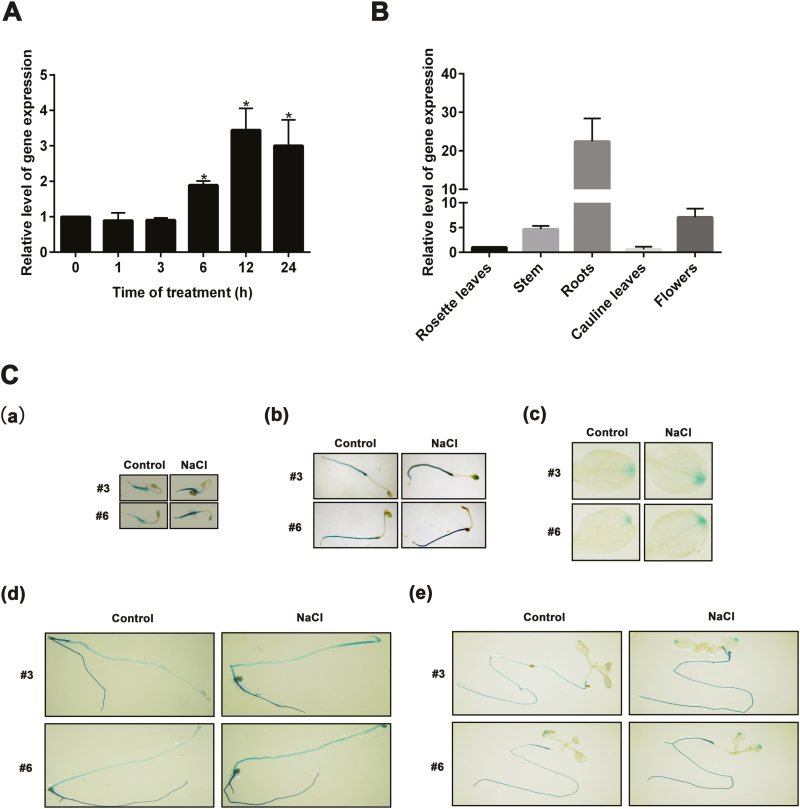
XTH30 expression was examined in order to investigate whether it responds to salt stress. As shown in Fig. 1A, the expression of XTH30 was strongly induced at 6 h, maximal at 12 h, and then decreased at 24 h after 150 mM NaCl treatment. The XTH30 expression in various tissues was also investigated. The results showed that XTH30 accumulated at high levels in root, stem, and flower but at low levels in rosette leaves and cauline leaves (Fig. 1B). Besides that, the 2.0 kb promoter region of XTH30 was fused to a β-glucuronidase (GUS) reporter gene. GUS activity was strongly detected in root and etiolated hypocotyl but weakly observed in leaves, which was further increased by NaCl treatment (Fig. 1C). These results are consistent with the qRT-PCR results.
Fig. 1.
Expression pattern of XTH30 in Arabidopsis. (A) Expression of XTH30 in Arabidopsis seedlings in response to salt stress. Fourteen-day-old seedlings were treated with 150 mM NaCl for different time as indicated. (B) Expression of XTH30 in rosette leaves, stem, roots, cauline leaves, and flowers. (C) XTH30 promoter::GUS expression patterns under salt stress in various developmental stages. XTH30 promoter::GUS expression of two lines (no. 3 and no. 6) in 3-day-old seedling (a), 5-day-old seedling (b), leaves (c), 7-day-old etiolated hypocotyl (d), and roots (e) exposed to salt stress. Seedlings in (a, b) were treated with 150 mM NaCl for 1 h. Seedlings in (c–e) were treated with 150 mM NaCl for 12 h. Error bars represent SD (n=3) in (A, B). ACTIN 2 acts as a reference standard. The asterisk in (A) indicates a significant difference compared with the control using unpaired Student’s t-test (*P<0.05). Experiment in (B) was performed at least three times with similar results. (This figure is available in color at JXB online.)
XTH30 negatively affects salt stress tolerance
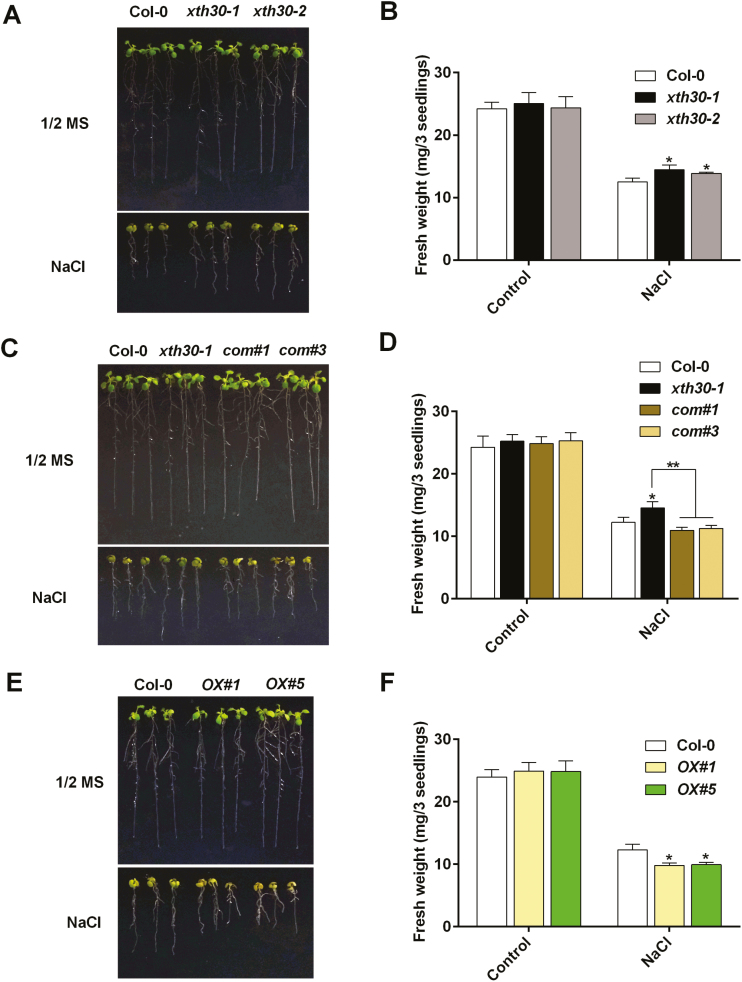
To determine the role of XTH30 in plants under salt stress, two independent mutants of XTH30 with T-DNA insertion (xth30-1 and xth30-2) were used to perform salt sensitivity assays. The T-DNA insertion in both xth30-1 and xth30-2 is located in the third intron of XTH30 genome and was confirmed by PCR using specific primers as indicated in Supplementary Fig. S1. When 5-day-old seedlings of the wild type and xth30 mutants were transferred to 1/2 MS medium with 125 mM NaCl, xth30 mutants showed more tolerance than the wild type (Fig. 2A). To completely investigate the function of XTH30, we expressed XTH30 in the xth30-1 knock-out background (com#1 and com#3) as well as in Col-0 (OX#1 and OX#5) (Supplementary Figs S2, S3). As shown in Fig. 2C, E, com#1 and com#3 were more sensitive to salt stress than xth30-1 and performed similarly to the wild type, and both OX#1 and OX#5 lines showed hypersensitivity to salt stress compared with the wild type. Hydroponically grown seedlings of xth30-1 and xth30-2 were also used to confirm the role of XTH30. The xth30 mutants wilted less and showed a much higher survival rate than the wild type under 125 mM NaCl treatment (Supplementary Fig. S4). These results indicate that XTH30 negatively affects salt stress tolerance. No growth phenotype difference was observed between xth30 mutants and the wild type under heavy metal stresses (CdCl2 and ZnSO4) or the plant hormone abscisic acid (ABA) treatment (Supplementary Fig. S5).
Fig. 2.
XTH30 negatively affects salt stress tolerance. (A) Analysis of salt sensitivity in xth30 mutants. Five-day-old seedlings of xth30-1, xth30-2, and Col-0 grown on 1/2 MS medium were transferred to 1/2 MS medium with or without 125 mM NaCl. Photographs were taken after 7 d of treatment. (B) Fresh weight of seedlings tested in (A). (C) Analysis of salt sensitivity in complementation of XTH30 in xth30-1 (com#1 and com#3). Five-day-old seedlings of xth30-1, complementation of XTH30 in xth30-1 (com#1 and com#3), and Col-0 grown on 1/2 MS medium were transferred to 1/2 MS medium with or without 125 mM NaCl. Photographs were taken after 7 d of treatment. (D) Fresh weight of seedlings tested in (C). (E) Analysis of salt sensitivity in XTH30 overexpressors (OX#1 and OX#5). Five-day-old seedlings of XTH30 overexpressors (OX#1 and OX#5) and Col-0 grown on 1/2 MS medium were transferred to 1/2 MS medium with or without 125 mM NaCl. Photographs were taken after 7 d of treatment. (F) Fresh weight of seedlings tested in (E). Data in (B, D, F) are means ±SD (n=3). The asterisks indicate a significant difference between various genotypes and wild type using unpaired Student’s t-test (*P<0.05, **P<0.01). (This figure is available in color at JXB online.)
To investigate the role of XTH30 in more detail, the seedling fresh weight of xth30, com, OX, and wild type was measured. As shown in Fig. 2B, D, F, compared with wild type, xth30 showed a higher seedling fresh weight, com showed similar performance, and OX showed a lower fresh weight. Salt stress results in both osmotic and ionic stress in plant (Munns and Tester, 2008). Next we determined whether salt tolerance of xth30 mutants is a specific response to the sodium ions. As shown in Supplementary Fig. S6, seedlings of xth30-1 and xth30-2 respond to Na+ and Li+, but not K+, Cl−, and osmotic stress, which was a similar behavior to sos mutants (Shi et al., 2000; Zhu, 2002). No significant difference was observed between the wild type and xth30 mutants in seed germination under salt stress (Supplementary Fig. S7) or in growth under normal conditions (Supplementary Fig. S8).
One of the most damaging effects of high salinity is the accumulation of salt in plants, and so Na+ content was investigated. The results showed that under 125 mM NaCl treatment, Na+ accumulated more in the wild type than in xth30 mutants in shoots, and there was no difference in Na+ accumulation between the wild type and xth30 mutants in roots (Supplementary Fig. S9). Salt stress leads to the overproduction of reactive oxygen species (ROS), which are highly reactive and toxic (Ma et al., 2012; Ben Rejeb et al., 2015). To understand the role of XTH30 in salt stress, we investigated H2O2 production in xth30 mutants and wild type seedlings under salt stress. When seedlings were grown on the 1/2 MS medium, the wild type and xth30 mutants showed a similar basal level of H2O2 (Supplementary Fig. S10). H2O2 levels increased both in the wild type and xth30 mutants after 125 mM NaCl treatment, and a relatively much smaller H2O2 increase was observed in xth30 mutants (Supplementary Fig. S10). We also measured the activities of antioxidant enzymes (catalase (CAT) and peroxidase (POD)) that are responsible for removing H2O2. As shown in Supplementary Fig. S11, the activities of CAT and POD increased both in the wild type and xth30 mutants exposed to NaCl treatment, and a greater increase was observed in xth30 mutants than in the wild type.
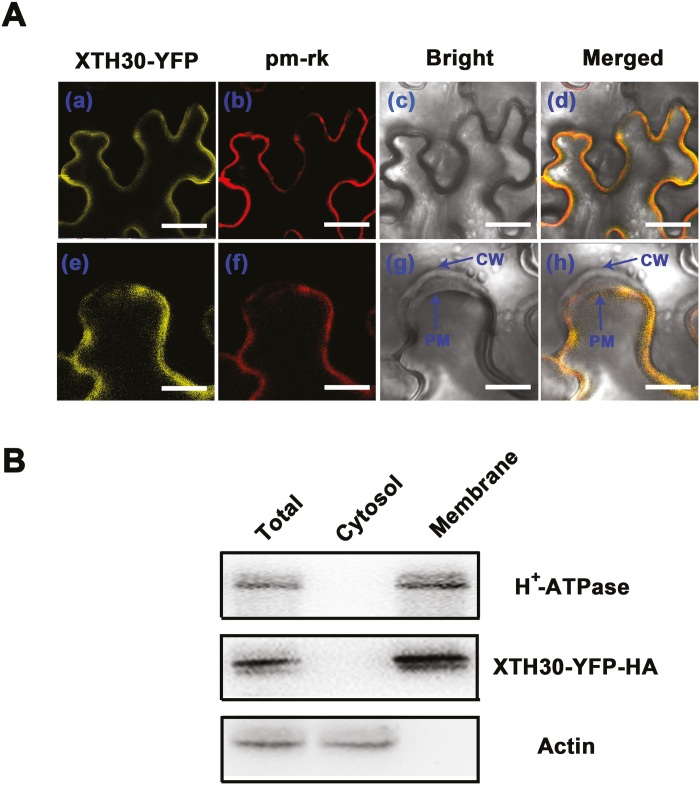
XTH30 is located in the plasma membrane
XTHs were shown to be localized to endoplasmic reticulum, cell wall, or plasma membrane (Genovesi et al., 2008; Zhu et al., 2012; Han et al., 2013). To understand the functions of XTH30, its subcellular localization was investigated. As shown in Fig. 3, XTH30–YFP protein was observed around the cell periphery consistent with plasma membrane-localized marker (pm-rk) (Nelson et al., 2007), suggesting that XTH30 was localized in plasma membrane (Fig. 3Aa–d). To clearly determine the plasma membrane location of XTH30, plasmolysis was performed. The results showed that XTH30 was localized in the plasma membrane but not in the cell wall (Fig. 3Ae–f). In order to further confirm the localization of XTH30, the plasma membrane and cytoplasmic fractions were isolated from XTH30 overexpressor (OX#1). The isolated plasma membrane fractions were confirmed by the marker, H+-ATPase. Consistent with the results of XTH30 expression in tobacco cell, XTH30–YFP–HA was only detected in plasma membrane (Fig. 3B).
Fig. 3.
Subcellular localization of XTH30. (Aa–d) Nicotiana benthamiana leaf cells co-expressing XTH30–YFP fusion protein (a), plasma membrane marker (pm-rk) (b), bright-field image (c), and with a merged image (d) in a non-plasmolysed tobacco cell. (Ae–h) Nicotiana benthamiana leaf cells co-expressing XTH30–YFP fusion protein (e), plasma membrane marker (pm-rk) (f), bright-field image (g), and with a merged image (h) in a plasmolysed tobacco cell by treatment with 300 mM mannitol for 10 min. Scale bars indicate 20 μm in (a–d), and 10 μm in (e–h). CW, cell wall; PM, plasma membrane. (B) Immunoblotting assay showing XTH30 localization. Two-week-old seedlings of OX#1, which expressed XTH30–YFP–HA fusion protein, were harvested. Plasma membrane and cytosol fractions were isolated, and the YFP–HA-tagged XTH30 was detected by HA antibody. Actin acts as a cytosol protein marker; H+-ATPase acts as a membrane protein marker. Similar results in (A, B) were from repeats of at least three times. (This figure is available in color at JXB online.)
XTH30 affects the xyloglucan oligosaccharide composition during salt stress
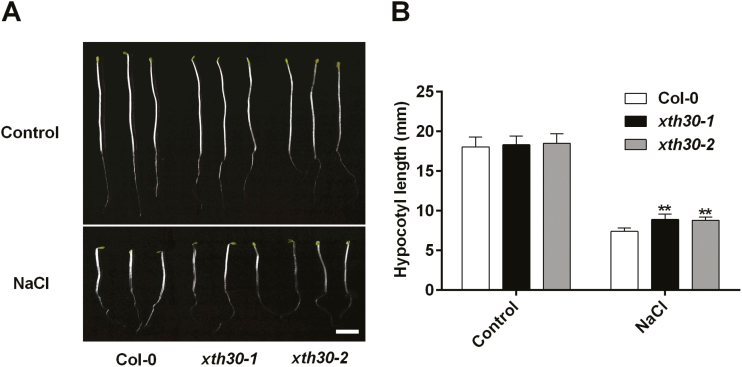
To further understand the role of XTH30 in plants under salt stress, etiolated hypocotyl of Arabidopsis seedling was used. It is widely used to study plant primary cell wall because no cell division occurs in the dark and growth is due to cell elongation only (Refrégier et al., 2004; Endler et al., 2015; Xiao et al., 2016). As shown in Fig. 4, the etiolated hypocotyl in xth30 mutants was longer than that in the wild type under salt stress. This indicated that XTH30 negatively modulated the salt tolerance during cell elongation in etiolated Arabidopsis seedlings.
Fig. 4.
XTH30 affects etiolated hypocotyl growth in response to salt stress. (A) Seedlings germinated and grown for 2 d on 1/2 MS plates and transferred to 1/2 MS plates supplemented with or without 100 mM NaCl and grown for an additional 5 d in the dark. (B) Quantification of hypocotyl elongation of seedlings in (A). Error bars represent SD; n>15 seedlings per biological replicate. Similar results were observed in three independent experiments. The asterisk indicates a significant difference between xth30 mutants and wild type using unpaired Student’s t-test (**P<0.01). Scale bar indicates 3 mm.
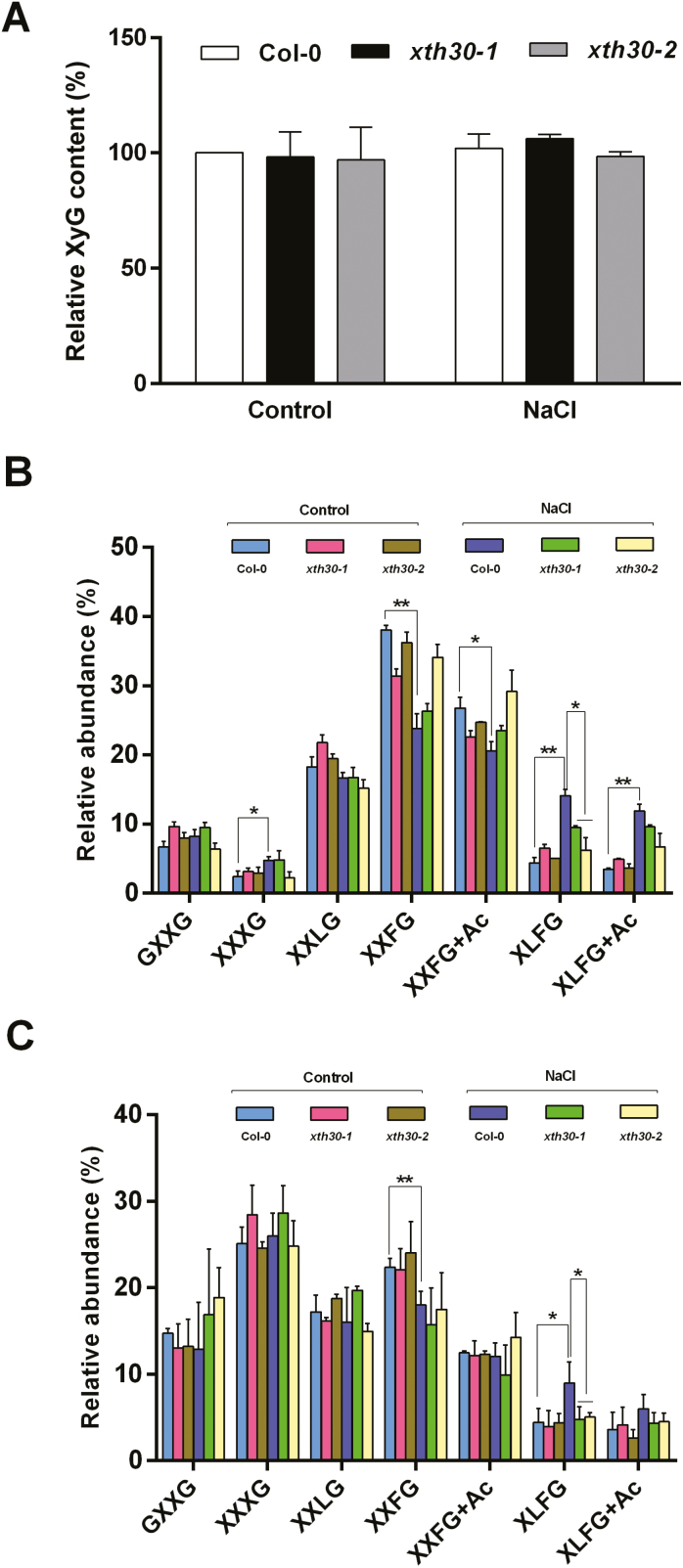
XTHs can cleave or rejoin XyG chains, resulting in the modification of content or structure of XyG (Nishitani and Tominaga, 1992), and XyG is shown to cross-link adjacent cellulose microfibrils and modulate cell wall extensibility in response to environmental variation (Hayashi et al., 2010), which prompted us to investigate the XyG content. Iodine staining (Kooiman, 1960; Gille et al., 2011; Vuttipongchaikij et al., 2012) was used to measure the amount of XyG in de-starched alkaline extracts from xth30 mutants and wild-type etiolated hypocotyls. Unexpectedly, the extractable XyG content in xth30 mutants was similar to that in the wild type after 100 mM NaCl treatment (Fig. 5A). These results suggest that XTH30 does not affect XyG content.
Fig. 5.
XTH30 affects XyG oligosaccharides in response to salt stress. (A) Extractable XyG content of hypocotyl quantified by iodine staining. Two-day-old etiolated seedlings were transferred to 1/2 MS plates containing 100 mM NaCl for another 5 d in the dark. The AIR was extracted from hypocotyl. Data are presented as percentage of the content of wild type (Col-0). Error bars represent SD (n=3). (B) XyG oligosaccharides generated from hypocotyl with xyloglucanase. (C) XyG oligosaccharides generated from hypocotyl with endoglucanase. Relative abundance of major subunits in (B, C) is shown as means of three biological replicates with SD. The asterisks indicate a significant difference between xth30 mutants and the wild type using unpaired Student’s t-test (*P<0.05, **P<0.01). (This figure is available in color at JXB online.)
Next we analysed the XyG structure from etiolated hypocotyls. Xyloglucan was treated with xyloglucanase, which attacks unconnected Glc residues and produces an oligosaccharide mixture that includes GXXG, XXXG, XXLG, XXFG, XXFG+Ac, XLFG, and XLFG+Ac (Gille et al., 2011). As shown in Fig. 5B, salt stress decreased the relative level of XXFG and XXFG+Ac, but increased the level of XXXG, XLFG, and XLFG+Ac in the wild type. More importantly, the increase of XLFG caused by salt stress in the wild type was partly blocked in xth30 mutants (Fig. 5B). To confirm the effect of XTH30 on XLFG content, another enzyme, endoglucanase (Sechet et al., 2016), was used. As expected, similar changes were also observed by digesting with endoglucanase (Fig. 5C). These results indicate that XTH30 plays an important role in the accumulation of the largely connected XyG oligosaccharides XLFG in response to salt stress.
XTH30 affects cellulose synthesis under salt stress
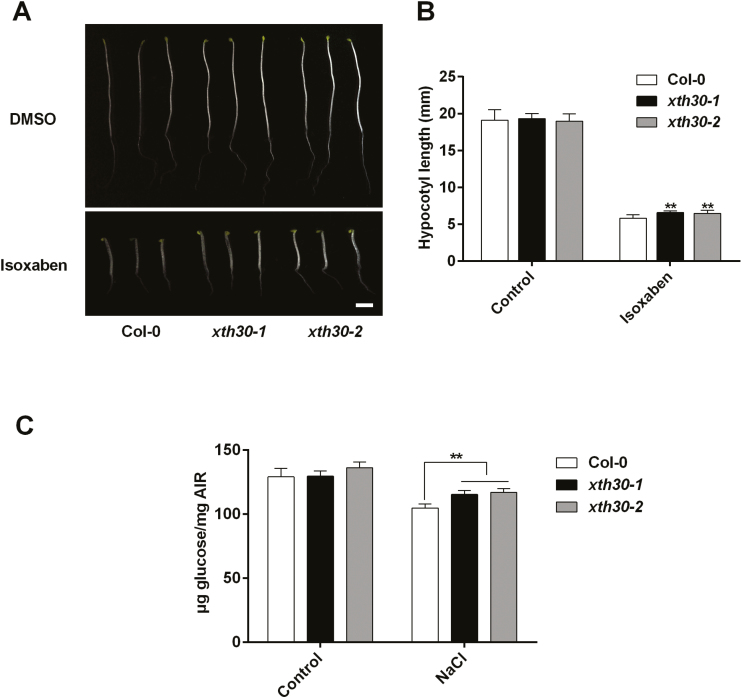
XyG affects cellulose biosynthesis (Xiao et al., 2016). To explore whether cellulose synthesis is also affected in xth30 mutants, we first used the cellulose synthesis inhibitor isoxaben (Lei et al., 2014; Zhang et al., 2016b). We grew the xth30 mutants and the wild type on 1/2 MS medium supplemented with isoxaben, and found that the xth30 mutants were less sensitive to growth inhibition by isoxaben than was the wild type (Fig. 6A, B). These results suggest that XTH30 might be involved in cellulose synthesis. Next, we estimated the crystalline cellulose content. Salt stress significantly decreased the crystalline cellulose content in the wild type (Fig. 6C), which is consistent with a previous report (Endler et al., 2015). The cellulose synthesis genes (CesA1, CesA3, CesA6) were then analysed by qRT-PCR. The results showed that salt stress increased CesA3 and CesA6 expression in both the wild type and xth30 mutants, which was not affected by the loss of XTH30 (Supplementary Fig. S12). However, the decrease of crystalline cellulose content resulting from salt stress was much smaller in xth30 mutants (Fig. 6C). These suggest that XTH30 affects cellulose synthesis under salt stress.
Fig. 6.
XTH30 affects cellulose synthesis under salt stress. (A) Seedlings germinated and grown for 6 d on 1/2 MS supplemented with or without 2 nM isoxaben in dark. Scale bar indicates 3 mm. (B) Quantification of hypocotyl elongation of seedlings in (A). Error bars represent SD, n>15 seedlings per biological replicate. Similar results were observed in three independent experiments. (C) Crystalline cellulose content of hypocotyl from xth30 mutants and wild type in response to salt stress. Error bars represent SD (n=3). The asterisks in (B, C) indicate a significant difference between xth30 mutants and wild type using unpaired Student’s t-test (**P<0.01).
XTH30 aggravates depolymerization of cortical microtubules under salt stress
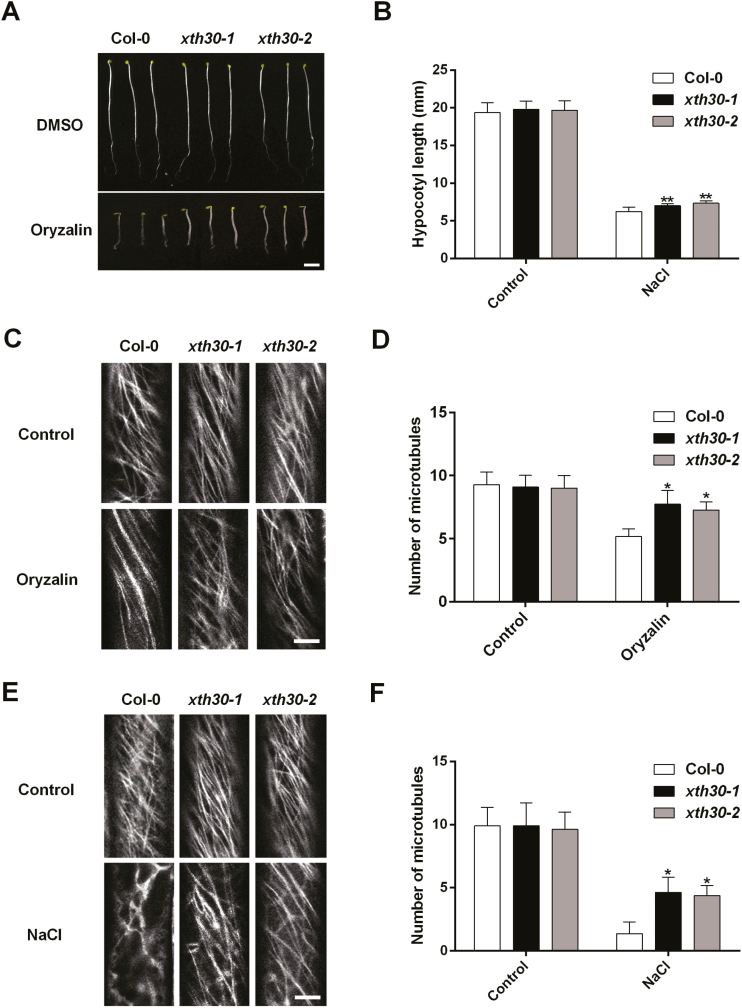
Cellulose microfibrils are typically transversely organized and can align with cortical microtubules that are tethered to the plasma membrane (Labavitch and Ray et al., 1974; Hayashi et al., 1984; Park and Cosgrove, 2015). Next, we analysed the effects of XTH30 on cortical microtubules. Oryzalin, a microtubule depolymerizing agent, was used (Endler et al., 2015), and the etiolated hypocotyl growth of xth30 mutants was less inhibited by oryzalin (Fig. 7A, B). Then, we generated transgenic lines that expressed the microtubules marker GFP–TUA6 (Arabidopsis α-tubulin 6 isoform fused to GFP) driven by the 35S promoter in the xth30 mutants and Col-0 to observe the cortical microtubule (Zhang et al., 2012). Treatment with 10 µM oryzalin triggered depolymerization of microtubules in the wild type and xth30 mutants, and the wild type showed more depolymerization than xth30 mutants (Fig. 7C, D). To further investigate the effect of XTH30 on cortical microtubules under salt stress, 3-day-old etiolated seedlings were transferred to 1/2 MS medium with or without 100 mM NaCl for 12 h. Under normal conditions, there is no obvious depolymerization of cortical microtubules in both xth30 mutants and the wild type (Fig. 7E, F). However, in the presence of NaCl, we observed obvious depolymerization of microtubules both in the wild type and xth30-1 mutants, and the wild type showed a more severe depolymerization compared with xth30 mutants (Fig. 7E, F). These results indicate that XTH30 aggravates depolymerization of cortical microtubules in response to salt stress.
Fig. 7.
Knockout of XTH30 alleviates depolymerization of cortical microtubules under salt stress. (A) Seedlings germinated and grown for 6 d on 1/2 MS with or without 400 nM oryzalin in dark. (B) Quantification of hypocotyl elongation of seedlings in (A). Error bars represent SD, n>15 seedlings per biological replicate. Similar results were observed in three independent experiments. (C) Confocal images of GFP–TUA6 labeled cortical microtubules. Three-day-old etiolated seedlings were exposed to 10 µM oryzalin for 5 min. The epidermal cells in etiolated hypocotyls were observed. (D) Quantification of hypocotyl elongation of seedlings in (C). (E) Confocal images of GFP–TUA6 labeled cortical microtubules. Three-day-old etiolated seedlings were exposed to 100 mM NaCl for 12 h. The epidermal cells in etiolated hypocotyls were observed. (F) Quantification of hypocotyl elongation of seedlings in (E). The number of cortical microtubules in (D, F) was determined by counting the microtubules across a fixed line (~10 µm) vertical to the orientation of the most cortical microtubules of the cell. Data in (D, F) represent the mean ±SD of three independent experiments with a minimum of 10 cells from three seedlings assessed in each experiment. Scale bar in (A) indicates 3 mm, and scale bar in (C, E) indicates 5 μm. The asterisks in (B, D, F) indicate a significant difference between xth30 mutants and wild type using unpaired Student’s t-test (*P<0.05, **P<0.01).
Discussion
As a structural layer outside the cell membrane, the plant cell wall not only provides the cell with structural support but also acts as the first line of defense against various stresses. The cell wall is flexible so that it can be rapidly remodeled when subjected to developmental, biotic, or abiotic stimuli. Maintaining the synthesis/deposition of cellulose is vital for the tolerance to salt stress. Several genes involved in cellulose synthesis (such as CesA1 and CesA6) as well as companions of cellulose synthase proteins (CC1 and CC2) have been found to function in salt stress responses, and knockout of these genes resulted in salt stress sensitivity (Endler et al., 2015; Zhang et al., 2016a). However, the mechanisms of cell wall function in plant response to stresses are largely unknown. In this study, we revealed that an XTH, XTH30, negatively affected salt tolerance.
XTH enzymes play a role in cell wall loosening and therefore can affect cell expansion. Unlike mutants of other members of the XTH family, such as XTH21, XTH27, XTH28, and XTH31 (Matsui et al., 2005; Liu et al., 2007; Kurasawa et al., 2009; Zhu et al., 2012), xth30 mutants do not have an obvious growth phenotype (Supplementary Fig. S8). However, xth30 mutants showed a very different performance from the wild type when subjected to salt stress. We found that XTH30 negatively affected salt tolerance based on the following evidence. First, knock-out mutations in XTH30 led to a salt-tolerant phenotype during growth of seedlings and etiolated hypocotyls (Figs 2A, B, 4; Supplementary Figs S4, S6). Second, overexpression of XTH30 enhanced sensitivity to salt stress (Fig. 2E, F). Cho et al. (2006) found that CaXTH3 participated in the protection of Arabidopsis mesophyll cells from salt stress via strengthening the cell wall layers. PeXTH positively modulated salt tolerance in tobacco by mainly affecting the development of leaf succulence (Han et al., 2013). XTH30 is another XTH member that is found to respond to salt stress (Cho et al., 2006; Choi et al., 2011; Han et al., 2013). However, these XTH genes (CaXTH3 and PeXTH) act as positive regulators in response to salt stress. The XTH enzymes encoded by numerous XTH genes could cleave or re-ligate the xyloglycan chains in the plant cell wall by its two reverse functions, xyloglucan endotransglucosylase (XET) activity and/or xyloglucan endohydrolase (XEH) (Thompson and Fry, 2001; Nishikubo et al., 2011). This implies that members of the XTH family might have different roles in the same process.
XTH can cleave or rejoin XyG chains and change the XyG size in the primary cell wall (Thompson and Fry, 2001; Nishikubo et al., 2011; Park and Cosgrove, 2015). XyG is the most abundant hemicellulose in the primary cell wall in dicots. XyG often undergoes turnover by wall-localized enzymes during cell elongation (Hayashi et al., 1984). In this study, we observed that XTH30 did not affect the extractable XyG content and the abundance of XyG oligosaccharides, i.e. XXXG, XXLG, and XXFG, under salt stress (Fig. 5). However, we found that loss-of-function of XTH30 obviously suppressed the strong increases in the abundance of XyG side chain, XLFG, in the wild type under salt stress (Fig. 5B, C). Loss of MUR3 resulted in shorter etiolated hypocotyl phenotype due to the altering of galactose depleted-xyloglucan (Tamura et al., 2005; Kong et al., 2015). The altered side chain of XyG in the cell wall is more deleterious to cellular processes than the complete absence of xyloglucan (Xu et al., 2017). The salt-tolerant phenotype of xth30 mutants might be due to the relatively stable XyG side chain. This is also similar to the report that the mur3-1 mutant with altered XyG side chain is hypersensitive to salt stress (Li et al., 2013).
XyG binds tightly to cellulose within the primary cell wall, coating most available cellulose surfaces during their synthesis and tethering adjacent microfibrils to major load-bearing networks in the growing cell wall (Labavitch and Ray et al., 1974; Hayashi et al., 1984; Park and Cosgrove, 2015). Overexpression of AtXTH21 in Arabidopsis enhanced the deposition of cellulose (Liu et al., 2007). Expressing ZmXTH1 in Arabidopsis showed alterations in the cellulose content (Genovesi et al., 2008). In this study, we found that XTH30 affected the cellulose content independent of modulating the expression of cellulose biosynthesis genes (CesA1, CesA3, CesA6) in response to salt stress (Fig. 6; Supplementary Fig. S12). It is possible that XTH30 somehow directly affects the synthesis/deposition of the cellulose at the plasma membrane in response to salt stress. Cellulose microfibrils are typically transversely organized and can align with cortical microtubules that are tethered to the plasma membrane (Park and Cosgrove, 2015). Microtubule depolymerization and reorganization are believed to be essential for plant survival under salt stress (Wang et al., 2011; Zhang et al., 2012; Dou et al., 2018). XTH30 affected the cortical microtubule depolymerization in response to salt stress (Fig. 7), which contradicted the view that microtubules mass was inversely correlated with the cellulose crystallinity and CSC velocity (Fujita et al., 2011). However, there are also some studies indicating that inhibiting cellulose synthase activity with isoxaben could influence microtubule organization (Paredez et al., 2008; Scheible et al., 2011). XyG side chains in the xyloglucan xylosyltransferase mutant xxt affected cellulose synthase activity and cellulose content in primary cell walls, and disrupted microtubule stability (Xiao et al., 2016).
Interestingly, knock-out mutations of XTH30 led to lower Na+ accumulation in shoots (Supplementary Fig. S9) and showed similar Na+ accumulation in roots compared with the wild type, which suggests that XTH30 affected Na+ transportation from root to shoot. Based on the lower Na+ content and higher antioxidant defense ability, knock-out mutations of XTH30 led to a lower level of H2O2 than that of the wild type under salt stress (Supplementary Figs S10, S11), which alleviated the damage of salt stress to plants. The mechanism needs to be further explored.
Thus, our findings suggest that the modified XyG side chain detected in xth30 mutants potentially affects cellulose synthesis and cortical microtubule stability through its effects on the interactions with components of the cell wall under salt stress (Supplementary Fig. S13).
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Summary of T-DNA lines used in this study.
Fig. S2. The expression of XTH30 in wild type, xth30-1, and complementation of XTH30 in xth30-1.
Fig. S3. The expression of XTH30 in wild type and XTH30 overexpressors.
Fig. S4. Sensitivity of hydroponically grown seedlings of xth30 mutants and wild type to salt stress.
Fig. S5. XTH30 does not affect the tolerance to heavy metal stress (Zn2+ and Cd2+) and ABA.
Fig. S6. xth30 mutants are resistant to Na+ and Li+.
Fig. S7. XTH30 does not affect the germination of seeds exposed to salt stress.
Fig. S8. The phenotypes of wild type and xth30 mutants.
Fig. S9. Effect of XTH30 on Na+ accumulation in hydroponically grown seedlings exposed to salt stress.
Fig. S10. Accumulation of H2O2 in wild-type and xth30 mutant seedlings.
Fig. S11. Effect of XTH30 on the activities of antioxidant enzymes exposed to salt stress.
Fig. S12. The effect of XTH30 on the expressions of CesA1, CesA3, and CesA6 in response to salt stress.
Fig. S13. A potential model for XTH30 regulation in salt stress responses.
Table S1. Primer sequences used in this study.
Accession numbers
Sequences data from this article relate to the following IDs:
ACTIN 2, AT3G18780; CesA1, At4g32410; CesA3, At5g05170; CesA6, At5g64740; XTH30, AT1G32170.
Acknowledgements
We are grateful to ABRC for providing Arabidopsis seed stock. This study was supported by grants from the National Natural Science Foundation of China (31671603); the Natural Science Foundation of Jiangsu Province (BK20161450); the Fundamental Research Funds for the Central Universities (KYZ201637); Six Talent Peaks Program of Jiangsu Province (2016-NY-079) and Postgraduate Research and Practice Innovation Program of Jiangsu Province. Work conducted by the Joint BioEnergy Institute was supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research under contract no. DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the US Department of Energy.
References
- Ben Rejeb K, Lefebvre-De Vos D, Le Disquet I, Leprince AS, Bordenave M, Maldiney R, Jdey A, Abdelly C, Savouré A. 2015. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytologist 208, 1138–1148. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hong X, Zhang H, Wang Y, Li X, Zhu JK, Gong Z. 2005. Disruption of the cellulose synthase gene, AtCesA8/IRX1, enhances drought and osmotic stress tolerance in Arabidopsis. The Plant Journal 43, 273–283. [DOI] [PubMed] [Google Scholar]
- Cho SK, Kim JE, Park JA, Eom TJ, Kim WT. 2006. Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Letters 580, 3136–3144. [DOI] [PubMed] [Google Scholar]
- Choi JY, Seo YS, Kim SJ, Kim WT, Shin JS. 2011. Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/hydrolase, enhanced tolerance to salt and drought stresses without phenotypic defects in tomato plants (Solanum lycopersicum cv. Dotaerang). Plant Cell Reports 30, 867–877. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. 2005. Growth of the plant cell wall. Nature Reviews. Molecular Cell Biology 6, 850–861. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. 2015. Plant expansins: diversity and interactions with plant cell walls. Current Opinion in Plant Biology 25, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou L, He K, Higaki T, Wang X, Mao T. 2018. Ethylene signaling modulates cortical microtubule reassembly in response to salt stress. Plant Physiology 176, 2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A, Kesten C, Schneider R, Zhang Y, Ivakov A, Froehlich A, Funke N, Persson S. 2015. A mechanism for sustained cellulose synthesis during salt stress. Cell 162, 1353–1364. [DOI] [PubMed] [Google Scholar]
- Fang L, Ishikawa T, Rennie EA, et al. . 2016. Loss of inositol phosphorylceramide sphingolipid mannosylation induces plant immune responses and reduces cellulose content in Arabidopsis. The Plant Cell 28, 2991–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Aldington S, Hetherington PR, Aitken J. 1993. Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiology 103, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Himmelspach R, Hocart CH, Williamson RE, Mansfield SD, Wasteneys GO. 2011. Cortical microtubules optimize cell-wall crystallinity to drive unidirectional growth in Arabidopsis. The Plant Journal 66, 915–928. [DOI] [PubMed] [Google Scholar]
- Genovesi V, Fornalé S, Fry SC, et al. . 2008. ZmXTH1, a new xyloglucan endotransglucosylase/hydrolase in maize, affects cell wall structure and composition in Arabidopsis thaliana. Journal of Experimental Botany 59, 875–889. [DOI] [PubMed] [Google Scholar]
- Gille S, de Souza A, Xiong G, Benz M, Cheng K, Schultink A, Reca IB, Pauly M. 2011. O-Acetylation of Arabidopsis hemicellulose xyloglucan requires AXY4 or AXY4L, proteins with a TBL and DUF231 domain. The Plant Cell 23, 4041–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang W, Sun J, et al. . 2013. Populus euphratica XTH overexpression enhances salinity tolerance by the development of leaf succulence in transgenic tobacco plants. Journal of Experimental Botany 64, 4225–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kaida R. 2011. Functions of xyloglucan in plant cells. Molecular Plant 4, 17–24. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kaida R, Kaku T, Baba K. 2010. Loosening xyloglucan prevents tensile stress in tree stem bending but accelerates the enzymatic degradation of cellulose. Russian Journal of Plant Physiology 57, 316–320. [Google Scholar]
- Hayashi T, Wong YS, Maclachlan G. 1984. Pea xyloglucan and cellulose: II. Hydrolysis by pea endo-1,4-β-glucanases. Plant Physiology 75, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XS, Wang W, Zhang Q, Liu JH. 2013. A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiology 162, 1178–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkowska MM, Testerink C. 2015. Tuning plant signaling and growth to survive salt. Trends in Plant Science 20, 586–594. [DOI] [PubMed] [Google Scholar]
- Kong Y, Peña MJ, Renna L, et al. . 2015. Galactose-depleted xyloglucan is dysfunctional and leads to dwarfism in Arabidopsis. Plant Physiology 167, 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooiman P. 1960. A method for the determination of amyloid in plant seeds. Recueil des Travaux Chimiques des Pays-Bas 79, 675–678. [Google Scholar]
- Kurasawa K, Matsui A, Yokoyama R, Kuriyama T, Yoshizumi T, Matsui M, Suwabe K, Watanabe M, Nishitani K. 2009. The AtXTH28 gene, a xyloglucan endotransglucosylase/hydrolase, is involved in automatic self-pollination in Arabidopsis thaliana. Plant & Cell Physiology 50, 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch JM, Ray PM. 1974. Turnover of cell wall polysaccharides in elongating pea stem segments. Plant Physiology 53, 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Lee HG, Yoon S, Kim HU, Seo PJ. 2015. The Arabidopsis MYB96 transcription factor is a positive regulator of ABSCISIC ACID-INSENSITIVE4 in the control of seed germination. Plant Physiology 168, 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Zhang T, Strasser R, et al. . 2014. The jiaoyao1 mutant is an allele of korrigan1 that abolishes endoglucanase activity and affects the organization of both cellulose microfibrils and microtubules in Arabidopsis. The Plant Cell 26, 2601–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouxel O, Choo TS, Séveno M, Usadel B, Faye L, Lerouge P, Pauly M. 2002. Rapid structural phenotyping of plant cell wall mutants by enzymatic oligosaccharide fingerprinting. Plant Physiology 130, 1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Guan Q, Wang ZY, Wang Y, Zhu J. 2013. A bi-functional xyloglucan galactosyltransferase is an indispensable salt stress tolerance determinant in Arabidopsis. Molecular Plant 6, 1344–1354. [DOI] [PubMed] [Google Scholar]
- Liu YB, Lu SM, Zhang JF, Liu S, Lu YT. 2007. A xyloglucan endotransglucosylase/hydrolase involves in growth of primary root and alters the deposition of cellulose in Arabidopsis. Planta 226, 1547–1560. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F. 2012. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na⁺/K⁺ homeostasis in Arabidopsis under salt stress. Journal of Experimental Botany 63, 305–317. [DOI] [PubMed] [Google Scholar]
- Matsui A, Yokoyama R, Seki M, Ito T, Shinozaki K, Takahashi T, Komeda Y, Nishitani K. 2005. AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. The Plant Journal 42, 525–534. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681. [DOI] [PubMed] [Google Scholar]
- Mutwil M, Debolt S, Persson S. 2008. Cellulose synthesis: a complex complex. Current Opinion in Plant Biology 11, 252–257. [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A. 2007. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. The Plant Journal 51, 1126–1136. [DOI] [PubMed] [Google Scholar]
- Nishikubo N, Takahashi J, Roos AA, Derba-Maceluch M, Piens K, Brumer H, Teeri TT, Stålbrand H, Mellerowicz EJ. 2011. Xyloglucan endo-transglycosylase-mediated xyloglucan rearrangements in developing wood of hybrid aspen. Plant Physiology 155, 399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R. 1992. Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. The Journal of Biological Chemistry 267, 21058–21064. [PubMed] [Google Scholar]
- Paredez AR, Persson S, Ehrhardt DW, Somerville CR. 2008. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiology 147, 1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ. 2015. Xyloglucan and its interactions with other components of the growing cell wall. Plant & Cell Physiology 56, 180–194. [DOI] [PubMed] [Google Scholar]
- Pauly M, Keegstra K. 2016. Biosynthesis of the plant cell wall matrix polysaccharide xyloglucan. Annual Review of Plant Biology 67, 235–259. [DOI] [PubMed] [Google Scholar]
- Refrégier G, Pelletier S, Jaillard D, Höfte H. 2004. Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis. Plant Physiology 135, 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C. 2011. Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis ixr1 mutants. Proceedings of the National Academy of Sciences, USA 98, 10079–10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P. 2010. Hemicelluloses. Annual Review of Plant Biology 61, 263–289. [DOI] [PubMed] [Google Scholar]
- Sechet J, Frey A, Effroy-Cuzzi D, et al. . 2016. Xyloglucan metabolism differentially impacts the cell wall characteristics of the endosperm and embryo during Arabidopsis seed germination. Plant Physiology 170, 1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu JK. 2000. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences, USA 97, 6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YZ, Zhu XF, Miller JG, Gregson T, Zheng SJ, Fry SC. 2015. Distinct catalytic capacities of two aluminium-repressed Arabidopsis thaliana xyloglucan endotransglucosylase/hydrolases, XTH15 and XTH31, heterologously produced in Pichia. Phytochemistry 112, 160–169. [DOI] [PubMed] [Google Scholar]
- Strohmeier M, Hrmova M, Fischer M, Harvey AJ, Fincher GB, Pleiss J. 2004. Molecular modeling of family GH16 glycoside hydrolases: potential roles for xyloglucan transglucosylases/hydrolases in cell wall modification in the Poaceae. Protein Science 13, 3200–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Shimada T, Kondo M, Nishimura M, Hara-Nishimura I. 2005. KATAMARI1/MURUS3 is a novel Golgi membrane protein that is required for endomembrane organization in Arabidopsis. The Plant Cell 17, 1764–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhaken R. 2014. Cell wall remodeling under abiotic stress. Frontiers in Plant Science 5, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JE, Fry SC. 2001. Restructuring of wall-bound xyloglucan by transglycosylation in living plant cells. The Plant Journal 26, 23–34. [DOI] [PubMed] [Google Scholar]
- Vuttipongchaikij S, Brocklehurst D, Steele-King C, Ashford DA, Gomez LD, McQueen-Mason SJ. 2012. Arabidopsis GT34 family contains five xyloglucan α-1,6-xylosyltransferases. New Phytologist 195, 585–595. [DOI] [PubMed] [Google Scholar]
- Wang R, Jing W, Xiao L, Jin Y, Shen L, Zhang W. 2015. The rice high-affinity potassium Transporter1;1 is involved in salt tolerance and regulated by an MYB-type transcription factor. Plant Physiology 168, 1076–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kurepa J, Hashimoto T, Smalle JA. 2011. Salt stress-induced disassembly of Arabidopsis cortical microtubule arrays involves 26S proteasome-dependent degradation of SPIRAL1. The Plant Cell 23, 3412–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, McFarlane HE, Persson S. 2016. The impact of abiotic factors on cellulose synthesis. Journal of Experimental Botany 67, 543–552. [DOI] [PubMed] [Google Scholar]
- Xiao C, Zhang T, Zheng Y, Cosgrove DJ, Anderson CT. 2016. Xyloglucan deficiency disrupts microtubule stability and cellulose biosynthesis in Arabidopsis, altering cell growth and morphogenesis. Plant Physiology 170, 234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wang M, Shi D, Zhou G, Niu T, Hahn MG, O’Neill MA, Kong Y. 2017. DGE-seq analysis of MUR3-related Arabidopsis mutants provides insight into how dysfunctional xyloglucan affects cell elongation. Plant Science 258, 156–169. [DOI] [PubMed] [Google Scholar]
- Yan J, Guan L, Sun Y, Zhu Y, Liu L, Lu R, Jiang M, Tan M, Zhang A. 2015. Calcium and ZmCCaMK are involved in brassinosteroid-induced antioxidant defense in maize leaves. Plant & Cell Physiology 56, 883–896. [DOI] [PubMed] [Google Scholar]
- Yokoyama R, Nishitani K. 2001. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant & Cell Physiology 42, 1025–1033. [DOI] [PubMed] [Google Scholar]
- Yokoyama R, Rose JK, Nishitani K. 2004. A surprising diversity and abundance of xyloglucan endotransglucosylase/hydrolases in rice. Classification and expression analysis. Plant Physiology 134, 1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lin F, Mao T, Nie J, Yan M, Yuan M, Zhang W. 2012. Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. The Plant Cell 24, 4555–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SS, Sun L, Dong X, Lu SJ, Tian W, Liu JX. 2016a Cellulose synthesis genes CESA6 and CSI1 are important for salt stress tolerance in Arabidopsis. Journal of Integrative Plant Biology 58, 623–626. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nikolovski N, Sorieul M, et al. . 2016. b Golgi-localized STELLO proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis. Nature Communications 7, 11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. 2002. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. 2016. Abiotic stress signaling and responses in plants. Cell 167, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XF, Shi YZ, Lei GJ, et al. . 2012. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. The Plant Cell 24, 4731–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yan J, Liu W, et al. . 2016. Phosphorylation of a NAC transcription factor by a calcium/calmodulin-dependent protein kinase regulates abscisic acid-induced antioxidant defense in maize. Plant Physiology 171, 1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.