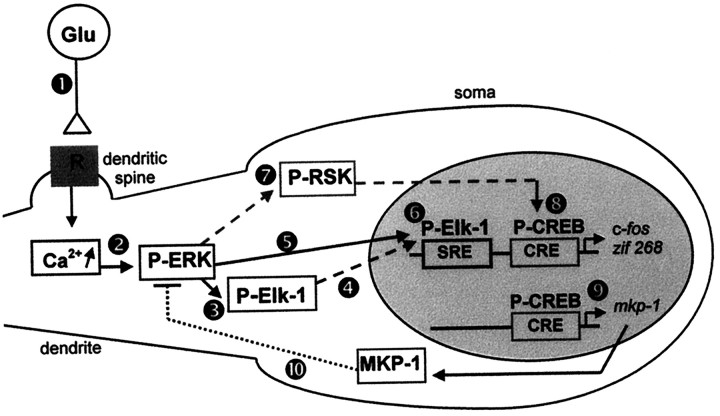
Fig. 9.
Proposed model for ERK-dependent IEG induction on corticostriatal pathway stimulation. Cortical fibers impinge specifically on dendritic spines of striatal neurons (Smith and Bolam, 1990). The electrical stimulation of these afferents leads to glutamate release at corticostriatal synapses (Reubi and Cuenod, 1979), which in turn act via glutamatergic receptors (1). The subsequent and local elevation of intracellular calcium levels, via NMDA receptors, activates ERK machinery (2) located in the dendrites (Fiore et al., 1993a; Ortiz et al., 1995). Elk-1 proteins that are present in dendrites (Sgambato et al., 1998) are phosphorylated locally by ERK proteins (3). Because both ERK and Elk-1 hyperphosphorylated proteins also are found in the nucleus, we suggest that they are translocated into the nucleus on activation (4, 5). In the nucleus Elk-1 can mediate the transcriptional regulation of SRE-containing IEGs like c-fos and zif 268(6). In parallel, we show that activated ERK proteins lead to a hyperphosphorylation of the nuclear CREB protein. The activation of the CREB kinase RSK, a cytoplasmic substrate of ERK proteins (Strugill et al., 1988) (7), and its subsequent translocation into the nucleus (Chen et al., 1992) could explain the ERK-dependent CREB activation in our model. In the nucleus CREB mediates the transcriptional regulation of CRE-containing IEGs like c-fos, zif 268(8), and MKP-1 (9). The protein MKP-1 may act in a negative feedback loop (10) to downregulate ERK activation. Thus, two different ERK modules are critically involved in IEG induction: ERK/Elk-1/SRE and ERK/?/CREB/CRE.