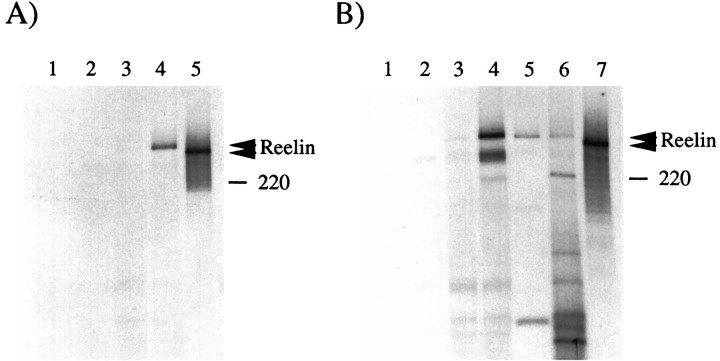
Fig. 5.
Reelin is modified and secreted by COS or cerebellar cells. A, COS cells (lanes 1–4) were transfected with (lanes 3, 4) or without (lanes 1, 2) pCrlM. The [35S]-labeled supernatant was subjected to immunoprecipitation with no antibody (lanes 1, 3) or with anti-Myc antibody 9E10 (lanes 2, 4). A specific band of ∼400 kDa (top arrowhead) was detected only in the sample containing both pCrlM and anti-Myc. The predicted 385 kDa in vitro translated [35S]-labeled product of pCrlM was immunoprecipitated with the anti-Myc antibody (lane 5). The protein produced in vitro (bottom arrowhead) seems to have a lower molecular mass than that obtained in COS cells. B, The supernatant from COS cells transfected with no DNA (lanes 1, 2) or pCrlM (lanes 3, 4), cerebellar cell supernatant (lane 5) or cerebellar cell lysates (lane 6), and in vitro translation reaction of pCrlM (lane 7) were labeled with [35S], as described in Materials and Methods, and subjected to immunoprecipitation with the CR-50 antibody. All immunoprecipitates from transfected COS or cerebellar cells migrated at a higher apparent molecular weight (top arrowhead) than the in vitro translated product (bottom arrowhead). The position of a 220 kDa molecular weight marker is indicated.