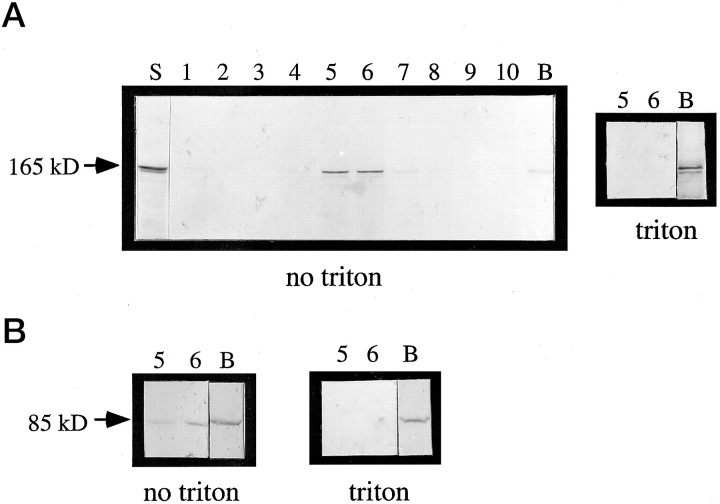
Fig. 6.
The Cat-307 protein cofractionates with microsomal membranes and with β-COP. A crude microsomal fraction prepared by differential centrifugation (Malhotra et al., 1989) contains the Cat-307 protein (A, lane S). The microsomal fraction was then further purified in a step sucrose gradient (A, lanes 1-10,B). A, Western blot analysis shows that the protein recognized by Cat-307 comigrates on a step sucrose density gradient with microsomes (no triton, fractions 5 and 6). Little immunoreactivity is detected at the bottom of the gradient (B, no triton). However, after treatment of the microsomes with Triton X-100 before centrifugation, the Cat-307 protein is retained in the bottom fraction of the gradient (B,triton). Triton X-100 solubilizes microsomal membranes; thus, the lack of floatation of the Cat-307 protein in the presence of Triton X-100 indicates that the floatation in the gradient in the absence of Triton X-100 is attributable to an association of the protein with intact microsomal membranes. B, Western blot analyses of β-COP shows an identical fractionation pattern to that observed for the Cat-307 protein; that is, in the absence of Triton X-100, immunoreactivity for β-COP is seen in fractions 5 and 6, thereby confirming the association of the Cat-307 protein with the microsomal membrane fractions. Furthermore, pretreatment of the microsomal membranes with 1% Triton X-100 prevents the floatation of β-COP in the sucrose density gradient; thus, immunoreactivity for β-COP is no longer detected in fractions 5 and 6 but is seen exclusively at the bottom of the gradient.