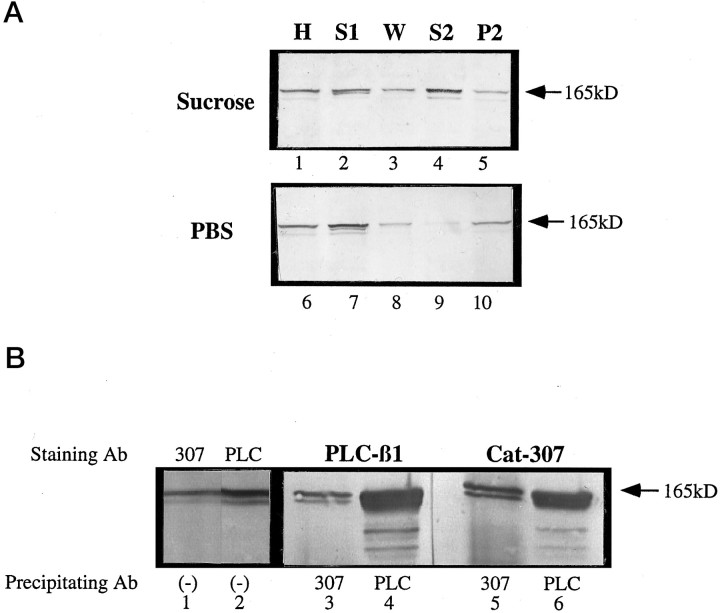
Fig. 7.
The Cat-307 protein is PLC-β1. A, Cortical tissue was homogenized in either 320 mm sucrose (lanes 1-5) or PBS (lanes 6–10). The homogenates (H, lanes 1 and 6) were spun at 100,000 ×g for 1 hr and separated into supernatant (S1, lanes 2 and 7) and pellet fractions. The pellet fraction was washed in an equal volume of the appropriate buffer and spun at 100,000 × g. The supernatant fraction was removed (W, lanes 3 and 8) and the pellet rehomogenized in an equal volume of PBS. The H was allowed to sit for 20–30 min at 4°C and was then centrifuged for 1 hr. The supernatant (S2,lanes 4 and 9) and pellet (P2, lanes 5 and 10) fractions were collected, and all fractions were subjected to Western blot analysis for the Cat-307 protein. The large amount of Cat-307 protein in the S2 fraction of the sucrose homogenates compared with the PBS homogenates indicates that the Cat-307 protein is associated with the microsomal membranes in a noncovalent manner that is dependent on the osmolarity of the homogenizing solution. This type of membrane association is characteristic of peripheral membrane proteins and is identical to that shown previously for the COPs. The Cat-307 protein was further concentrated and purified from the S2 fraction by ammonium sulfate precipitation and FPLC using a monoQ column (data not shown). Amino acid sequence analysis identified the Cat-307 protein as PLC-β1. B, Immunoblot of purified Cat-307 protein stained with antibodies to Cat-307 (lane 1) or PLC-β1 (lane 2). One lane of the purified protein was transferred to nitrocellulose, cut in half, and stained for the two antibodies. Cat-307 and antibody to PLC-β1 each identifies an exactly comigrating protein doublet. Proteins immunoprecipitated with either PLC-β1 (lanes 4 and 6) or Cat-307 (lanes 3 and 5) are immunoreactive for both Cat-307 (lanes 5 and6) and PLC-β1 (lanes 3 and4). Each antibody immunoprecipitates a protein recognized by the other antibody, indicating that the Cat-307 protein is PLC-β1.