Fig. 1.
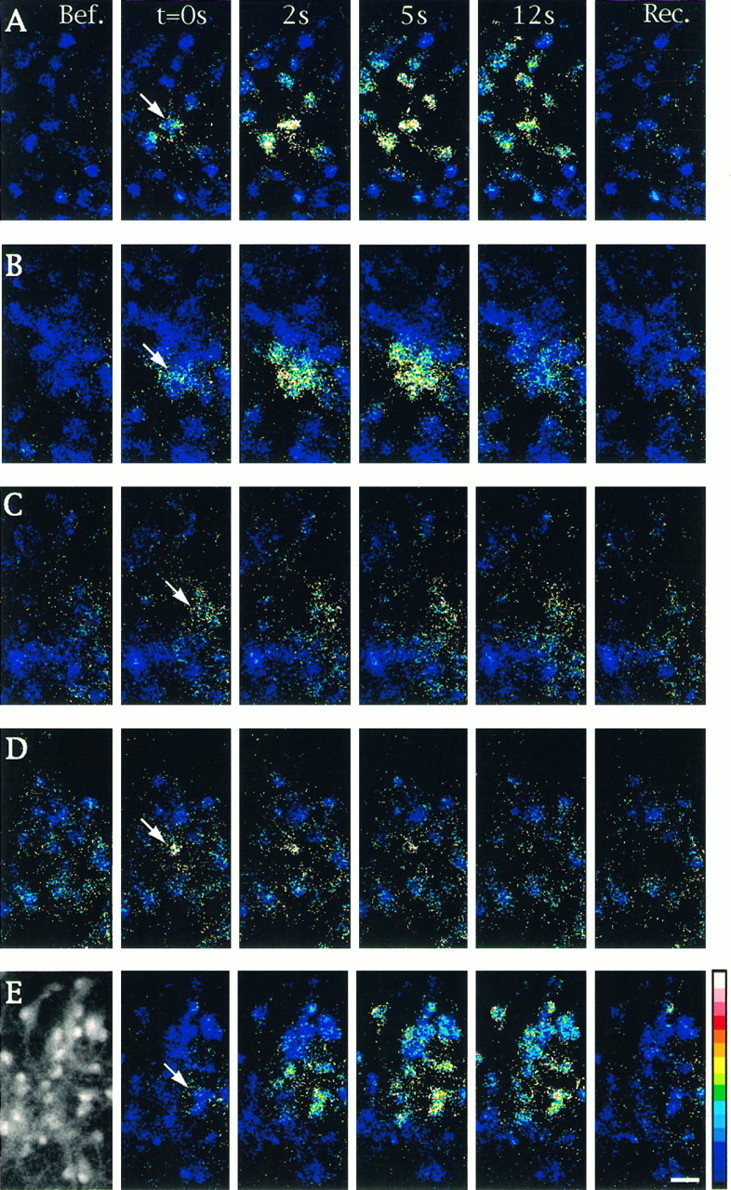
Properties of intercellular calcium signaling induced by focal application of ionomycin in cultured astrocytes. Changes in [Ca2+]i were monitored by calcium imaging in cells loaded with Indo1-AM. Unitary cell stimulations were achieved by brief pressure application of ionomycin (50 μm) in the close vicinity of an astrocyte located in the center of the microscopic field (arrows). Sequential pseudocolor representations of [Ca2+]i are expressed as the ratio of Indo1-AM emissions (F405/F480 nm) caused by the excitation at 355 nm. The number of astrocytes per field was usually ∼30, as shown in the example of the fluorescent image taken at emission at 480 (left-hand side inE). Shown is a sequence of six pseudocolor images taken before (Bef.), at the indicated times after stimulation (t = 0s, 2s, 5s,12s), and at recovery (Rec.) when [Ca2+]i returned to resting level (150 to 180 sec). A, Under control conditions, most of the cells present in the field responded to stimulation. B, In the presence of the gap junction blocker 18α-GA (10 μm), only a few cells exhibited a [Ca2+]i increase. C, The PLC inhibitor U73122 (5 μm) reduced the response in the stimulated cell and inhibited propagation of intercellular calcium waves. D, Thapsigargin (2 μm) application blocked intercellular calcium signals when ionomycin was applied after recovery of the initial [Ca2+]i increase evoked by this compound. E, In the presence of dantrolene (10 μm), an inhibitor of Ca2+-induced Ca2+ release, no significant changes were observed in the extent of intercellular calcium signaling when compared with control. Pseudocolor scale refers to ratios from 0.01 to 1.00, which corresponded to estimated [Ca2+]i values of 10–1200 nm, respectively. Calibration bar, 25 μm.
