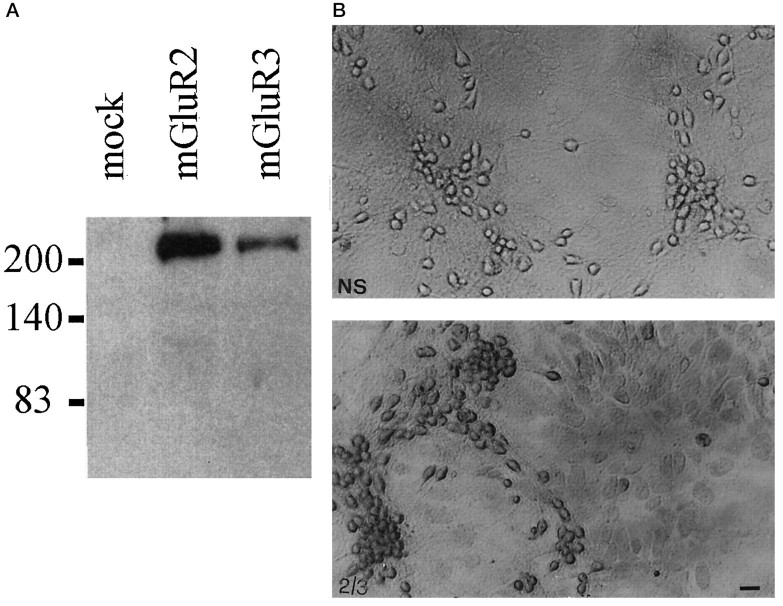
Fig. 6.
A, Immunoblotting with purified mGluR2/3 antibodies on lysates from HEK 293 cells transfected with a plasmid encoding the mGlu2 or -3 receptor. Note that the antibody reacts mostly with a high molecular weight band, which may correspond to receptor aggregates (Hayashi et al., 1993). A 100 kDa band corresponding to the deduced molecular weight of mGlu2 and -3 receptors was detected only after long-term exposure. Untransfected HEK 293 cells (mock) or HEK 293 cells transfected with mGlu1a, -5a, and -4 receptor cDNA (not shown) were not stained by the mGlu2/3 receptor antibody. B, Immunostaining of mixed cultures of cortical cells with the mGlu2/3 receptor antibody. Note that both neurons and astrocytes are stained by the antibody. 2/3, Specific staining; NS, nonspecific staining. Scale bar, 20 μm. We also performed Western blot analysis on protein extracts from membranes prepared from (1) pure cultures of mouse astrocytes, (2) pure cultures of rat astrocytes, (3) mouse cerebral cortex, and (4) rat cerebral cortex (60 μg of protein loaded for each lane). In extracts from mouse or rat cerebral cortex, the mGlu2/3 receptor antibody labeled exclusively a 100 kDa band and an additional band of higher molecular weight, which may correspond to receptor aggregates (not shown). No immunolabeling was observed in extracts from either mouse or rat cultured astrocytes. This suggests that the antibody that we have used is specific for mGlu2/3 but the amount of receptor(s) expressed by cultured astrocytes is possibly too low to be detected by Western blot analysis.