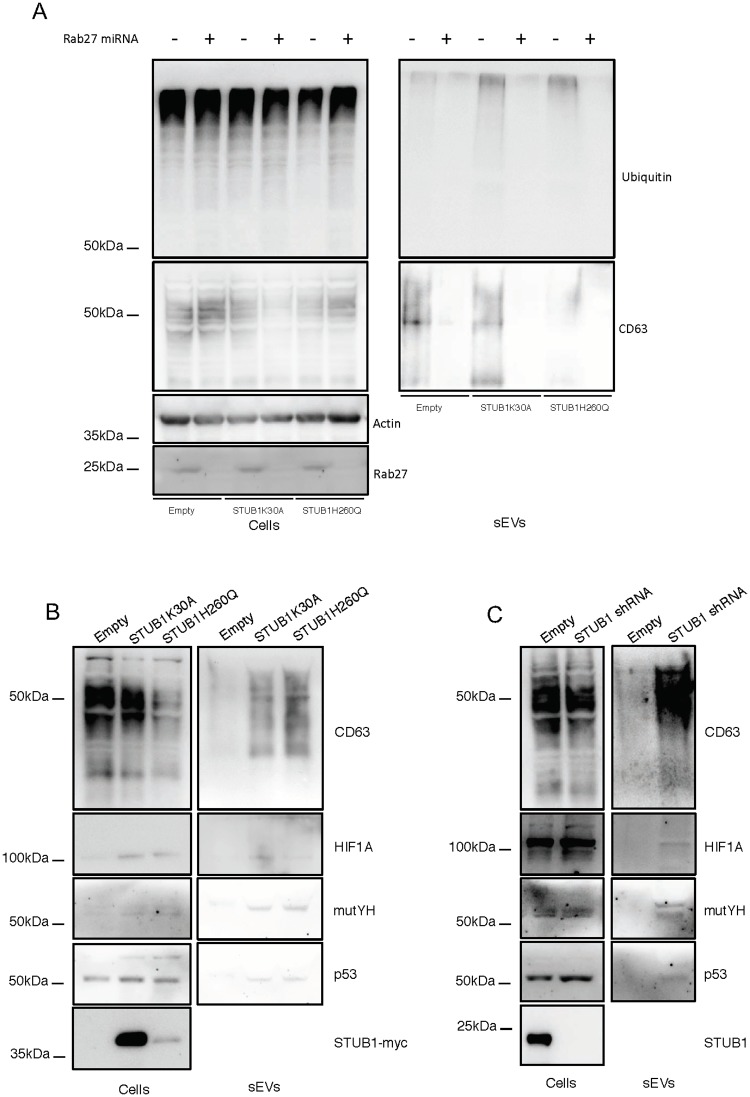
Fig 5. Rab27 depletion prevents the secretion of ubiquitinated proteins by sEVs and STUB1 inactivation leads to an increase of proteasomal substrates in secreted sEVs.
ARPE-19 cells were transduced using lentiviral particles containing vectors for the expression of either STUB1K30A or STUB1H260Q, with adenoviral particles containing shRNA against STUB1 or with adenoviral particles containing miRNA against Rab27. Control cells were transduced with an empty vector. A) Western blot of whole cell lysates and sEVs samples with antibodies against ubiquitin, CD63 and GAPDH shows that depletion of Rab27 prevents the secretion of vesicles loaded with ubiquitinated proteins. B,C) Western blot of whole cell lysates and sEVs sample with antibodies against CD63, HIF1A, mutYH and p53. The expression of STUB1-DN mutants, as well as, the depletion of STUB1 increases the presence of proteasomal substrates in released sEVs. All samples were analyzed under the same experimental conditions.