Figure 5.
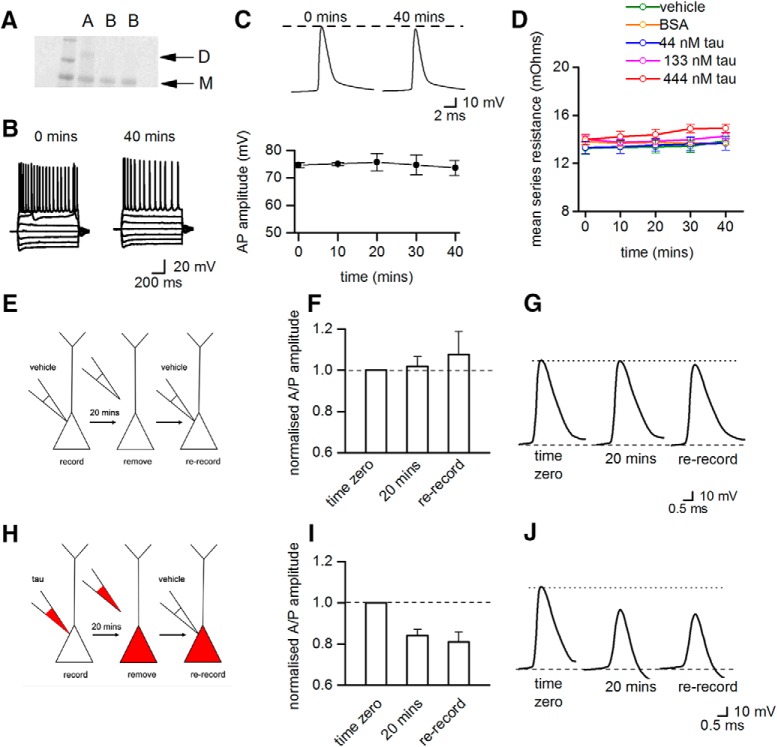
Monomeric tau protein does not change action potential dynamics and the accumulation of oTau in the pipette cannot account for the effects on action potential dynamics. A, Gel showing dimeric tau protein (lane A) and tau protein converted to monomers (mTau, in lanes labeled B). B, There is no significant difference between current−voltage responses from a hippocampal CA1 neurons injected with 444 nM mTau measured at time 0 and after 40 min of recording. C, Top panel shows examples of action potential waveforms recorded at time 0 and after 40 min of recording from a neuron injected with 444 nM mTau. The mTau does not change the amplitude or kinetics of the action potential waveform. C, Bottom panel summarizes data from five neurons, showing that action potential amplitude does not change over time when neurons are injected with 444 nM mTau. D, The mean series resistance plotted against time for neurons injected with vehicle, BSA, or oTau (44, 133, or 444 nM). There was no significant difference in series resistance between treatments and the series resistance did not significantly increase during recordings. E, Diagram illustrating the control re-patching protocol. Pyramidal-cell recordings were made (20 min) with vehicle present in the intracellular solution. The patch-electrode was carefully removed from the cell and then the cell was re-recorded from using a new patch-electrode also containing vehicle. F, There was no change in action potential amplitude during the initial recording (at 20 min, action potential amplitude was 102 ± 4% of the amplitude at time 0) and after re-recording with a new electrode (108 ± 11% of amplitude at time 0, n = 4 neurons). Thus, the re-patching protocol itself does not induce changes in action potential amplitude. G, Examples of average action potential waveforms from a single recording. H, Diagram illustrating the test re-patching protocol. Pyramidal cell recordings were made (20 min) with oTau (444 nM) in the intracellular solution. The patch-electrode was then carefully removed from the cell and the cell was then re-recorded from using a patch-electrode containing intracellular solution with vehicle. I, In cells injected with oTau (444 nM), action potential amplitude decreased during the initial recording period (at 20 min, action potential amplitude was 84 ± 3.1% of the amplitude at time 0) and remained depressed following re-recording with a new patch-electrode containing vehicle (81 ± 5% of amplitude at time 0, n = 3). J, Shows examples of average action potential waveforms from a single re-patching experiment with 444 nM oTau. These control experiments strongly suggest that the aggregation of oTau in the electrode or in the area around the initial whole-cell breakthrough does not account for the observed changes in action potential dynamics. Figure Contributions: Emily Hill performed the experiments and analyzed the data.