Abstract
Background
Knowing patients' ulcerative colitis history is essential to selecting the appropriate therapy according to risk stratification.
Objective
To evaluate and identify predictive factors of non-response to aminosalicylates judged as the need for a step-up approach over time.
Methods
A case-control study of ulcerative colitis patients treated with aminosalicylates after the diagnosis of disease flare included in the ENEIDA single-centre registry from 1997 to 2017. Long-term treatment maintenance with aminosalicylates and higher therapeutic requirements were recorded. The cumulative incidence of treatment escalation was estimated using Kaplan-Meier curves and compared by the log-rank test. Cox regression analysis was performed to identify predictive factors of treatment with immunomodulators, biological agents or surgery.
Results
A total of 457 patients were included, of whom 28% (n = 126) were non-responders to aminosalicylates. The cumulative probability for a step-up approach within 20 years of follow up was 35%, mainly due to steroid-dependent colitis. Risk factors for treatment escalation were age ≤27 years (hazard ratio 2.31, 95% confidence interval 1.36–3.92), extensive colitis (hazard ratio 1.65, 95% confidence interval 1.04–2.60), Mayo endoscopic subscore ≥2 (hazard ratio 1.45, 95% confidence interval 1.02–2.06) and extraintestinal manifestations (hazard ratio 2.04, 95% confidence interval 1.03–4.05).
Conclusions
Aminosalicylates represent an effective maintenance therapy. Younger age, extensive colitis, endoscopic disease severity and extraintestinal manifestations are risk factors for higher therapeutic requirements.
Keywords: Colitis, ulcerative, aminosalicylic acids, treatment outcome
Key summary
Current knowledge
With ever-increasing options for ulcerative colitis treatment, the appropriate time for a step-up approach and change in maintenance therapy is difficult to assess.
Data on long-term course of the disease judged by the need for a step-up approach to higher therapeutic requirements (immunosuppressive therapy or surgery) among patients in remission with aminosalicylates are scarce.
What is new?
Non-response to aminosalicylates is low (28%) and among patients managed with a step-up approach, 79% will require treatment escalation before 5 years of disease duration.
Younger age, extensive colitis, endoscopic disease severity and presence of extraintestinal manifestations at disease diagnosis are predictive factors of higher therapeutic requirements.
Introduction
The clinical course of ulcerative colitis (UC) is variable and marked by periods of exacerbation and remission. First-line maintenance treatment is considered 5-aminosalicylate (5-ASA) due to its beneficial effect in preventing relapse.1,2 The use of immunomodulators (IMM) and biological agents has increased over time.3 With ever-increasing options, the appropriate time for a step-up approach and change of maintenance therapy has become more difficult to assess.
Recognizing patients at risk of medical therapy failure can appropriately optimize treatment escalation for better control of the disease based on risk stratification. Response to steroids and outcomes such as colectomy rate or time to relapse have been well studied.4–6 Little is known about the long-term course of the disease judged by the need of treatment escalation among patients in remission with 5-ASA. Such data would represent a real-life scenario that controlled trials are unlikely to provide.
The main aim of the present study was to evaluate long-term efficacy of 5-ASA maintenance treatment, judged as no need for a step-up approach to higher therapeutic requirements (IMM, biological therapy or colectomy). The secondary aim was to identify risk factors for treatment escalation.
Materials and methods
Study design and patient population
An observational, retrospective, single-centre, case-control study was performed. All consecutive patients with UC followed in our Inflammatory Bowel Disease (IBD) unit from January 1997 until January 2017 were screened for eligibility. Inclusion criteria were: firm UC diagnosis; age ≥ 18 years; patients subsequently treated with 5-ASA after diagnosis of disease flare; first outbreak of inflammatory activity treated with steroids and/or 5-ASA; minimum follow up of 1 year. Exclusion criteria were: history of 5-ASA hypersensitivity; loss of follow up; first outbreak of inflammatory activity treated with IMM, biological agents or colectomy; and surgery indicated for dysplasia or colon cancer. Informed consent to participate in our database was obtained from all patients. The study was approved by the institutional ethics committee of the hospital.
Description of variables
Variables collected on the first visit were: gender, age at diagnosis, disease extension, endoscopic disease severity, 5-ASA dose and administration regime (oral, topical, combination), hospitalization, family history of IBD, extraintestinal manifestations (EIMs), nonsteroidal anti-inflammatory drug consumption and smoking habits (active, ex-smoker, never smoker). Patient-disease data recorded during follow up included: disease extension, extent progression, endoscopic progressive features (pseudopolyposis, colonic stenosis, loss of haustral folds, bridging fibrosis), EIMs, EIM appearance, smoking habits, time since diagnosis (disease duration) and treatment escalation (date of step-up approach).
Definitions
UC diagnosis (based on clinical, endoscopic and histological criteria) and steroid-dependent colitis were defined according to the European Crohn's and Colitis Organisation consensus guidelines.7 Remission was clinically defined as cessation of rectal bleeding, urgency and increased stool frequency, not necessarily confirmed by endoscopic mucosal healing. Satisfactory response to 5-ASA was considered when no treatment escalation was needed during follow up. Step-up approach group included patients treated with IMM (mercaptopurine, azathioprine, methotrexate), biological agents (anti-TNF, vedolizumab) or surgery (colectomy). Patients who received immunosuppressive therapy or surgery were assigned to the case group and patients who remained on 5-ASA (topical, oral or combined, not concomitant to other therapies) were assigned to the control group. The baseline 5-ASA dose was the first maintenance dosage initiated after remission of diagnosis disease flare. Disease extent was determined with colonoscopy and classified according to Montreal classification (E1–E3).8 Patients were categorized at diagnosis and with follow up (higher E-number at study closure). Endoscopic disease severity was assessed according to the endoscopic Mayo subscore. This score was only included if it was explicitly documented in the endoscopic report or could be determined by description of mucosal disease severity. The EIMs included peripheral arthropathy, ankylosing spondylitis, erythema nodosum, iritis, episcleritis and primary sclerosing cholangitis.
Data collection and follow up
Data were obtained from our single-centre ENEIDA project established database (reference code 0028). The ENEIDA Registry is a prospectively maintained database of IBD patients, initiated in 2006. Patients diagnosed before 2006 were retrospectively included at the time of ENEIDA initiation and are prospectively updated. This registry includes clinical, demographic, endoscopic and therapeutic data. Patients were followed from the date of diagnosis until the date of the last visit, study closure, or death. Treatment regimen along the follow-up period was not the same in all patients. Biological therapy approval or 5-ASA maintenance dose adjustment has changed the medical management during the study period. Different therapeutic attitudes at different times were considered with a pre- and post-biologic era analysis. Since 2012, the treatment regimen has been based on Spanish-developed guidelines9 that are in agreement with European guidelines.
Statistical analysis
Qualitative variables were expressed as frequencies and quantitative variables as the median and interquartile range (IQR). Differences between groups were evaluated using Mann-Whitney U test for continuous data and the chi-square or Fisher test for categorical data, as required. Time was calculated from the day of 5-ASA initiation to the date of censoring: date of first exposure to treatment escalation, study closure or death, whichever came first. Time to event analysis was conducted using the Kaplan-Meier method. Survival curves were compared using the log-rank test to identify variables at diagnosis, potentially associated with a step-up approach during follow up. Multivariate Cox proportional hazard regression analyses were performed to determine the independent contribution of each factor on time to treatment escalation. Variables with a p value < 0.10 were introduced in the multivariate model. Because topical treatment is restricted to proctitis, the disease extension and 5-ASA administration regime can be considered confounding factors. Consequently, the 5-ASA administration regime was not included in the Cox regression model. Receiver operating characteristic curve analysis was performed to select the optimum cut-off value of age for classifying the non-response to 5-ASA. As the date of censoring was only determined with first exposure to treatment escalation, multivariate logistic regression analysis was performed to evaluate factors associated with different step-up approaches individually. The results are presented as estimated hazard ratios (HR) or odds ratios (OR) with respective 95% confidence interval (95%CI) and p values. All tests were two sided and a p value < 0.05 was considered statistically significant. Analyses were performed with the SPSS V22.0 software package.
Results
Patient outcomes
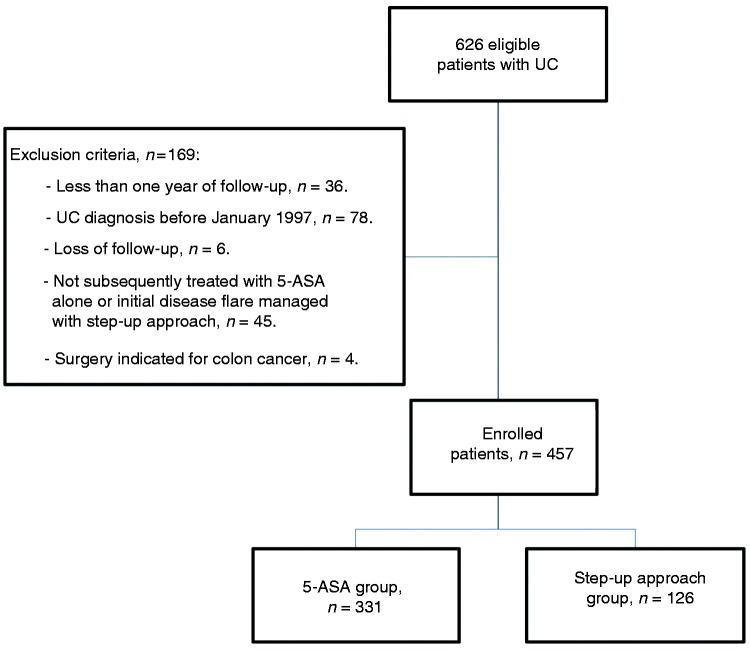
We identified 457 patients treated with 5-ASA after initial disease flare, in whom 72% (n = 331) achieved a satisfactory response (Figure 1). From initial eligible population, 7% of the patients were not included in the study because diagnosis of disease flare was treated with immunosuppressive therapy or colectomy. Table 1 shows the study population characteristics. Among the step-up approach group, steroid-dependent colitis was the main reason for treatment escalation (76%; n = 96). Other causes for treatment escalation included: steroid-refractory colitis (13%), EIMs (5%) and other (5%). Higher therapeutic requirements included: 97% (n = 122) IMM, 57% (n = 72) biological agents and 13% (n = 16) colectomy, with a predominant branch of IMM and biological therapy combination (48%). The Kaplan-Meier curve of the whole study cohort showed a cumulative probability for treatment escalation of 7%, 23% and 30% at 1, 5 and 10 years respectively (Figure 2). Cumulative probability to remain free of higher therapeutic requirements after 20 years of follow up was of 65%. Looking at the step-up approach group, median time to treatment escalation was 2 years from diagnosis, and after 5 years of follow-up 79% were already managed with treatment escalation.
Figure 1.
Study protocol flowchart.
Table 1.
Characteristics of study population according to treatment requirements.
| Patients’ characteristics | Total cohort (n = 457) | 5-ASA group (n = 331) | Step-up approach group (n = 126) | p value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Gender: female, n (%) | 212 (46) | 157 (47) | 55 (44) | 0.47 |
| Age at diagnosis, median (IQR) | 37 (26–48) | 38 (27–48) | 33 (25–48) | 0.02 |
| 5-ASA dose (mg), median (IQR) | 3 (2–3) | 3 (2–3) | 3 (2–3.6) | 0.02 |
| 5-ASA administration regime, n (%) | <0.01 | |||
| - Topical | 37 (8) | 34 (10) | 3 (3) | <0.01 |
| - Oral | 199 (44) | 152 (46) | 47 (37) | 0.09 |
| - Combined | 221 (48) | 145 (44) | 76 (60) | <0.01 |
| Disease extent, n (%) | <0.01 | |||
| - E1, proctitis | 132 (29) | 105 (32) | 27 (21) | 0.03 |
| - E2, left-sided | 159 (35) | 121 (36) | 38 (30) | 0.20 |
| - E3, extensive | 166 (36) | 105 (32) | 61 (49) | <0.01 |
| Mayo endoscopic subscore, median (IQR) | 2 (1–2) | 1 (1–2) | 2 (1–2) | <0.01 |
| Family history of IBD, n (%) | 58 (13) | 44 (13) | 14 (11) | 0.53 |
| NSAID consumption, n (%) | 95 (22) | 68 (21) | 27 (22) | 0.93 |
| Smoking habit, n (%) | 0.19 | |||
| - Active smoker | 64 (14) | 52 (16) | 12 (10) | |
| - Ex-smoker | 109 (24) | 75 (23) | 34 (27) | |
| - Never smoker | 284 (62) | 204 (62) | 80 (64) | |
| EIMs, n (%) | 42 (9) | 20 (6) | 22 (18) | <0.01 |
| Hospitalization, n (%) | 17 (4) | 7 (2) | 10 (8) | <0.01 |
| Follow-up outcomes | ||||
| Disease extent, n (%) | <0.01 | |||
| - E1, proctitis | 87 (19) | 80 (24) | 7 (6) | <0.01 |
| - E2, left-sided | 156 (34) | 123 (37) | 33 (26) | 0.34 |
| - E3, extensive | 214 (47) | 128 (39) | 86 (68) | <0.01 |
| Disease extent progression, n (%) | 74 (16%) | 38 (12) | 36 (29) | <0.01 |
| Pseudopolyposis, n (%) | 55 (19) | 32 (15) | 23 (30) | <0.01 |
| Stenosis, n (%) | 10 (4) | 4 (2) | 6 (8) | 0.02 |
| Loss of haustral folds, n (%) | 25 (9) | 19 (9) | 6 (8) | 0.75 |
| Fibrous bridges, n (%) | 23 (8) | 18 (9) | 5 (7) | 0.57 |
| Smoking habit, n (%) | 0.59 | |||
| - Active smoker | 59 (13) | 48 (15) | 11 (9) | |
| - Ex-smoker | 136 (30) | 97 (29) | 39 (31) | |
| - Never smoker | 262 (57) | 186 (56) | 76 (60) | |
| EIMs, n (%) | 137 (30) | 78 (24) | 59 (47) | <0.01 |
| EIMs appearance, n (%) | 95 (21) | 58 (18) | 37 (29) | <0.01 |
| Follow-up time (years), median (IQR) | 9 (5–13) | 9 (4–13) | 10 (6–14) | <0.01 |
5-ASA: 5-aminosalicylate; EIM: extraintestinal manifestations; IBD: inflammatory bowel disease; IQR: inter-quartile range; NSAID: nonsteroidal anti-inflammatory drug.
Figure 2.
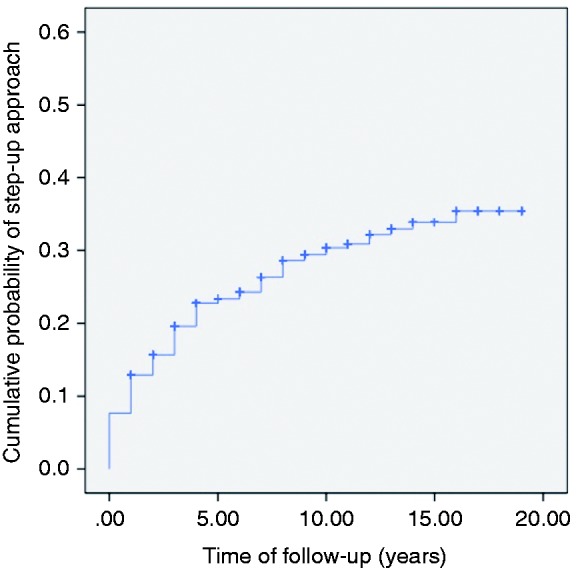
Kaplan-Meier plot showing the cumulative probability of step-up approach.
Time-to-treatment escalation: Survival curves and multivariate analysis
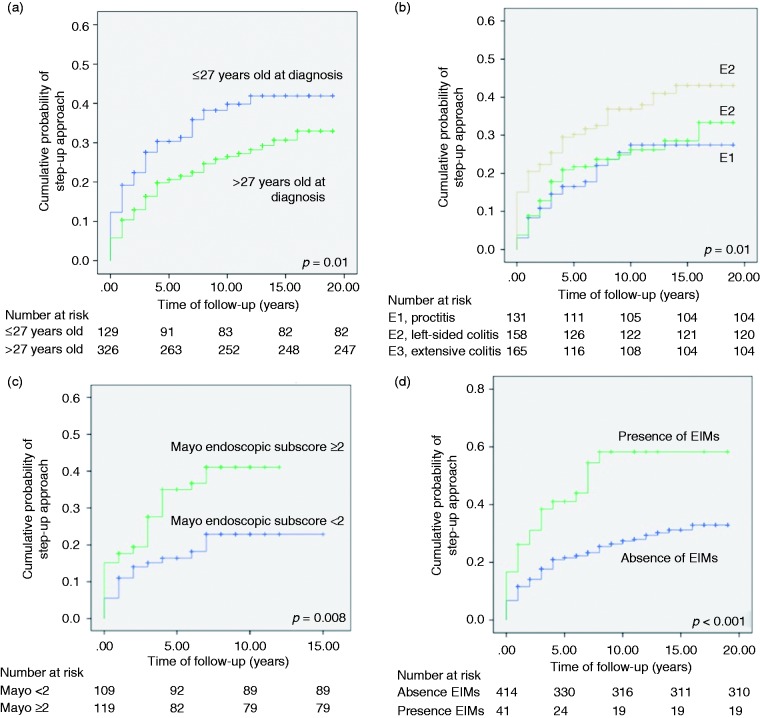
In the univariate analysis, age, 5-ASA dose and combined administration regime, extensive colitis, endoscopic disease severity, hospitalization and EIMs were significantly associated with higher therapeutic requirements. A multivariate Cox regression model for predicting the step-up approach controlling confounding effects was determined. Younger age, extensive colitis, Mayo endoscopic subscore ≥2 and EIMS were retained in the model as risk factors for treatment escalation (Table 2). The best classifying threshold for division of age groups was 27 years, with a raw sensitivity of 0.78 and specificity of 0.64 for therapeutic groups discrimination (p = 0.02). Cumulative incidence of treatment escalation after 3 years was 28% in the ≤ 27 years cohort vs 17% in the > 27 years group (Figure 3(a)). Although extensive colitis was associated with treatment escalation, no differences were identified between E1 and E2 (Figure 3(b)). The Kaplan-Meier curves for endoscopic disease severity (Mayo endoscopic subscore ≥ 2 was associated with a 45% risk increase of step-up approach) and EIMS, are illustrated in Figures 3(c)–(d).
Table 2.
Risk factors for non-response to 5-ASA within 20 years; multivariate Cox regression model analysis (HR 95%CI).
| Characteristics | HR (95%CI) | p value |
|---|---|---|
| Age at diagnosis | 2.31 (1.36–3.92) | <0.01 |
| Disease extent: | ||
| - E1, proctitis | Reference | |
| - E2, left-sided colitis | 1.24 (0.75–2.04) | 0.40 |
| - E3, extensive colitis | 1.65 (1.04–2.60) | 0.03 |
| Mayo endoscopic subscore | 1.45 (1.02–2.06) | 0.04 |
| Baseline 5-ASA dose | 0.92 (0.64–1.30) | 0.63 |
| Hospitalization | 1.68 (0.65–4.31) | 0.28 |
| EIMs | 2.04 (1.03–4.05) | 0.04 |
5-ASA: 5-aminosalicylate; CI: confidence interval; EIM: extraintestinal manifestations; HR: hazard ratio.
Figure 3.
Kaplan-Meier plot showing overall cumulative probability of step-up approach according to (a) age group (hazard ratio (HR) for treatment escalation in the ≤27 years old group, 2.31, 95% confidence interval (CI) 1.36–3.92; log rank = 0.010); (b) Montreal classification (HR for treatment escalation in the E3 group, 1.65, 95%CI 1.04–2.60; log rank = 0.012), (c) endoscopic disease severity (HR for Mayo endoscopic subscore ≥2, 1.45, 95% CI 1.02–2.06; log rank = 0.008) and (d) extraintestinal manifestations (EIMs) (HR for treatment escalation in the presence of EIMs group, 2.04, 95%CI 1.03–4.05; log rank < 0.001).
Outcomes based on different step-up approaches
Multivariate logistic regression analysis was performed to determine factors associated with different step-up approaches, analyzed individually and adjusted for sex and disease duration. Variables associated with the use of IMM and biological agents were younger age, extensive colitis, disease extent progression, endoscopic disease severity and EIMs. EIMs at diagnosis was associated with higher risk of IMM (OR 3.69, 95%CI 1.78–7.66) and biological therapy (OR 5.10, 95%CI 2.40–10.85), whereas appearance of EIMs during follow up (OR 2.15, 95%CI 1.20–3.86) and pseudopolyposis (OR 2.13, 95%CI 1.08–4.20) was only associated with IMM. Extensive colitis, previous exposure to IMM and steroid refractoriness were significantly associated with a higher risk of surgery. Distribution and association of disease extension and extent progression with different step-up approaches is shown in Table 3. Subgroup analysis was performed comparing therapeutic requirements in the pre- and post-biologic era, after infliximab approval for UC by the European Medicines Agency (February 2006). The post-biologic era group (n = 246) had a reduction in colectomy rates (7% vs 1%; p < 0.01). Among step-up approach group, only 21% were managed with treatment escalation after 5 years of follow up. In this cohort, a subgroup analysis was performed finding no differences between patients with short time to treatment escalation (≤5 years, n = 99) and long time to treatment escalation (>5 years, n = 27).
Table 3.
Multivariate logistic regression analysis showing association of disease extension and extent progression with different step-up approaches (expressed as: n (%); OR (95%CI); p value). Bold values indicate they are statistically significant.
| Disease extension | IMM (n = 122) | Biological therapy (n = 72) | Colectomy (n = 16) |
|---|---|---|---|
| E1 (proctitis) | Reference | Reference | n = 0 (0%) |
| E2 (left-sided) | n = 37 (30%); OR 2.07 (1.04–4.11); p = 0.04 | n = 21 (29%); OR 1.77 (0.80–3.70); p = 0.17 | Reference |
| E3 (extensive) | n = 58 (48%); OR 2.93 (1.45–5.89); p < 0.01 | n = 33 (46%); OR 2.74 (1.24–6.09); p = 0.02 | n = 15 (94%); OR 9.28 (1.14–75.31); p = 0.04 |
| Extent progression | n = 35 (47%); OR 5.19 (2.68–10.04); p < 0.01 | n = 23 (31%); OR 4.37 (2.15–8.89); p < 0.01 | n = 1 (2%); OR 0.63 (0.07–5.76); p = 0.68 |
CI: confidence interval; IMM: immunomodulators; OR: odds ratio.
Discussion
The present study addresses the efficacy of 5-ASA in clinical practice involving long-term follow up. Our main findings indicate that non-response to 5-ASA, judged as the need of higher therapeutic requirements, is low (28%) and can be predicted by easily assessed factors that may impact treatment strategies. To analyze stepwise-escalation of maintenance therapy, treatment efficacy was assessed among patients in clinical remission only under 5-ASA. Accordingly, 45 patients were excluded because the diagnosis of disease flare was managed with immunosuppressive therapy or surgery. However, if these patients were included in the analysis, the same predictive risk factors of 5-ASA non-response would have been obtained.
During the first 5–7 years of UC disease, cumulative risk of receiving IMM and biological therapy is 27–30% and 12–18% respectively in contemporary studies.10,11 With longer follow up (median disease duration of 9 years) and pre- and post-biologic population, we observed similar cumulative risk of 28% and 18% for IMM and biological therapy respectively. Similar percentages with a different follow-up time reflect that the step-up approach is mainly done during the first years of disease history. In our study, median time to treatment escalation was 2 years from diagnosis and after 5 years, 79% were already managed with a step-up approach. The 10-year cumulative risk of colectomy was 5%, lower than the resection rate reported in the pre-biologic era studies, but similar to the post-biologic Epi-IBD study.5,6,11 Surgery indicated for dysplasia or colon cancer was excluded in our study (n = 4) because we considered it a disease complication but not a non-response to treatment.
The definition of medical therapy failure in IBD is crucial for offering guidance on the treatment approach.12 Aggressive UC is defined as disease associated with a high relapse rate and the need for surgery.13 The efficacy of 5-ASA has been evaluated in patients who have never received immunosuppressive drugs, according to the aggressive disease definition. In terms of relapse, 5-ASA shows a satisfactory response rate of 25–50% after a median follow up of 50–118 months, with cumulative relapse rates after 1, 5 and 10 years of 22%, 60% and 80% respectively.14–16 With our study definition, a satisfactory response after a median follow up of 108 months was 72% and the cumulative probabilities for treatment escalation after 1, 5 and 10 years were 7%, 23% and 30%. Different percentages demonstrate that although the relapse rate is high, a low proportion of patients are managed with treatment escalation to prevent future relapse and cumulative side effects of steroids. In terms of surgery, previous studies in patients who have never received immunosuppressive drugs showed cumulative colectomy rates of 3%, 8% and 14% after 1, 3 and 5 years of follow up. 15,16 Our study, based on a larger cohort, showed lower cumulative rates: 2%, 3% and 4% after 1, 3 and 5 years.
Risk factors for relapse and colectomy have been previously published. Younger age, male, short disease duration, smoking status, extensive colitis and initial haemoglobin <10.5 g/dL are associated with clinical relapse in patients mainly treated with 5-ASA.4,14–17 Analyzing factors associated with step-up approach management, our data support the younger age and extensive colitis findings but, contrary to the relapse rate, no differences were observed for gender or smoking habits. However, a recent meta-analysis showed that disease flares were not significantly lower in smokers. 18 Association between age and 5-ASA non-response is consistent with other studies that observed a relationship between younger patients and a higher use of steroids or shorter time to relapse, 4,19 leading to a lower age for time to treatment escalation. In parallel with haemoglobin level and clinical relapse, the association between the Mayo endoscopic subscore and the step-up approach reflects that initial disease severity might be a significant predictor of poor outcomes.
It may be argued that when treatment escalation is mainly indicated due to steroid-dependent colitis, IMM are the first treatment option. Therefore, we performed subgroup analysis considering different step-up approaches individually confirming the same predictive risk for IMM and biological therapies. Evaluating risks for colonic extension, endoscopic progressive features and EIMs, our findings are in agreement with previous studies, providing new evidence about extent progression associated to biological therapy and EIM appearance related with IMM use.20–24 An important point is the association of age and immunosuppressive therapy use, differing from the Swiss IBD cohort registry and the ACCESS Group observations.20,25 In terms of surgery, extensive colitis, previous exposure to IMM, steroid refractoriness and disease diagnosis in the pre-biologic era were shown to impact the likelihood of the patient requiring a colectomy; these results are in keeping with previously published articles.5,6,25–27 Gender, smoking habits and hospitalization are known factors associated with colectomy risk.28,29 In our study, these variables showed univariate association but were not retained as a significant influence in the multivariate analysis. This might have occurred due to the small sample size when dividing patients into subgroups and to methodological definitions (smoking habits stratification). However, our results add valuable information to previous studies in patients under 5-ASA treatment after a first course of steroids.15,16
Our study has inherent limitations and strengths. Limitations included that the study was observational and retrospective, and thus prone to information bias and confounding variables. We attempted to control potential confounding by conducting subgroup and multivariate analyses. Moreover, follow up was longer among the step-up approach group and it may be argued that a proportion of patients categorized as 5-ASA responders would later require treatment escalation. To overcome this limitation, we performed a subgroup analysis including only patients with disease duration longer than 2 years (median time to treatment escalation), equalizing follow-up time between both therapeutic groups and confirming the results. With infliximab irruption, our cohort includes a differently managed population. Thus, we analysed differences in pre- and post-biologic periods, finding a reduction in colectomy rates after infliximab approval. In addition, we cannot affirm responders to 5-ASA were in clinical remission because disease activity variables (new flares, sporadic need of steroids) were not recorded. Steroid information addressed in the study is limited to treatment escalation indication. Our cohort included patients treated with 5-ASA after initial the disease flare, with no difference for whether this outbreak was managed with 5-ASA alone or combined with a first course of steroids. Furthermore, adherence to 5-ASA was not considered a possible risk factor for non-response. No serum data and faecal markers were collected because they were not performed in all patients.
There are also strengths for this study. Our cohort represents a large population followed up for long enough to assess the long-term disease course, with detailed phenotypic and endoscopic information. The major strength of our study lies in a hospital-based cohort avoiding selection bias and providing real-life information about patients' history.
In summary, we assessed 5-ASA efficacy considering step-up approach-free remission as a relevant surrogated marker. This new approach has practical implications: 1) 5-ASA is an effective maintenance therapy; a satisfactory response was achieved in 72% of the patients after 20 years of follow up; 2) among patients managed with a step-up approach, 79% will require treatment escalation before 5 years of disease duration; and 3) a younger age, extensive colitis, endoscopic disease severity and EIMs are associated with higher therapeutic requirements. These findings may be used in our clinical practice to identify patients that might benefit from an early step-up approach and minimize the morbidity associated with uncontrolled disease.
Acknowledgements
None to declare.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethics Approval
Study protocol was approved on 28 January 2016 by the Clinic University Hospital Ethical Committee (2000/007). The protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Informed Consent
Written informed consent was obtained from each patient included in the study.
References
- 1.Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-ASA in UC: A meta-analysis. Am J Gastroenterol 2011; 106: 601–616. [DOI] [PubMed] [Google Scholar]
- 2.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 2: Current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 3.Jeuring SF, Bours PH, Zeegers MP, et al. Disease outcome of ulcerative colitis in an era of changing treatment strategies: Results from the Dutch population-based IBDSL cohort. J Crohns Colitis 2015; 9: 837–845. [DOI] [PubMed] [Google Scholar]
- 4.Bitton A, Peppercorn MA, Antonioli DA, et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology 2001; 120: 13–20. [DOI] [PubMed] [Google Scholar]
- 5.Höie O, Wolters FL, Riis L, et al. Low colectomy rates in ulcerative colitis in an unselected European cohort followed for 10 years. Gastroenterology 2007; 132: 507–515. [DOI] [PubMed] [Google Scholar]
- 6.Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: Results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009; 44: 431–440. [DOI] [PubMed] [Google Scholar]
- 7.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 8.Satsangi J, Siverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006; 55: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomollón F, García-López S, Sicilia B, et al. Therapeutic guidelines on ulcerative colitis: A GRADE methodology based effort of GETECCU. Gastroenterol Hepatol 2013; 36: e1–47. [DOI] [PubMed] [Google Scholar]
- 10.Vester-Andersen MK, Prosberg MV, Jess T, et al. Disease course and surgery rates in inflammatory bowel disease: A population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol 2014; 109: 705–714. [DOI] [PubMed] [Google Scholar]
- 11.Burisch J, Katsanos KH, Christodoulou DK, et al. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort: An Epi-IBD study. J Crohns Colitis. Epub ahead of print 5 October 2018. DOI: 10.1093/ecco-jcc/jjy154. [DOI] [PubMed] [Google Scholar]
- 12.Volk N, Siegel CA. Defining failure of medical therapy for inflammatory bowel disease. Inflamm Bowel Dis 2019; 25(1): 74–77. [DOI] [PubMed] [Google Scholar]
- 13.Yarur AJ, Strobel SG, Deshpande AR, et al. Predictors of aggressive inflammatory bowel disease. Gastroenterol Hepatol 2011; 7: 652–659. [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HJ, Jung ES, Lee JH, et al. Long-term clinical outcomes and factors predictive of relapse after 5-aminosalicylate or sulfasalazine therapy in patients with mild-to-moderate ulcerative colitis. Hepatogastroenterology 2012; 59: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 15.Bello C, Belaiche J, Louis E, et al. Evolution and predictive factors of relapse in ulcerative colitis patients treated with mesalazine after a first course of corticosteroids. J Crohns Colitis 2011; 5: 196–202. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Planella E, Mañosa M, Van Domselaar M, et al. Long-term outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis 2012; 44: 206–210. [DOI] [PubMed] [Google Scholar]
- 17.Höie O, Wolters F, Riis L, et al. Ulcerative colitis: Patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol 2007; 102: 1692–1701. [DOI] [PubMed] [Google Scholar]
- 18.To N, Ford AC, Gracie DJ. Systematic review with meta-analysis: The effect of tobacco smoking on the natural history of ulcerative colitis. Aliment Pharmacol Ther 2016; 44: 117–126. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Cheon J, Moon C, et al. Do patients with ulcerative colitis diagnosed at a young age have more severe disease activity than patients diagnosed when older?. Digestion 2010; 81: 237–243. [DOI] [PubMed] [Google Scholar]
- 20.Ng SC, Zeng Z, Niewiadomski O, et al. Early course of inflammatory bowel disease in a population-based inception cohort study from 8 countries in Asia and Australia. Gastroenterology 2016; 150: 86–95. [DOI] [PubMed] [Google Scholar]
- 21.Hochart A, Gower-Rousseau C, Sarter H, et al. Ulcerative proctitis is a frequent location of paediatric-onset UC and not a minor disease: A population-based study. Gut 2017; 66: 1912–1917. [DOI] [PubMed] [Google Scholar]
- 22.Lau A, Chande N, Ponich T, et al. Predictive factors associated with immunosuppressive agent use in ulcerative colitis: A case-control study. Aliment Pharmacol Ther 2008; 28: 606–613. [DOI] [PubMed] [Google Scholar]
- 23.Vegh Z, Kurti Z, Gonczi L, et al. Association of extraintestinal manifestations and anaemia with disease outcomes in patients with inflammatory bowel disease. Scand J Gastroenterol 2016; 51: 848–854. [DOI] [PubMed] [Google Scholar]
- 24.Massinha P, Portela F, Campos S, et al. Ulcerative colitis: Are we neglecting its progressive character. GE Port J Gastroenterol 2017; 25(2): 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog D, Fournier N, Buehr P, et al. Age at disease onset of inflammatory bowel disease is associated with later extraintestinal manifestations and complications. Eur J Gastroenterol Hepatol 2018; 30(6): 598–607. [DOI] [PubMed] [Google Scholar]
- 26.Samuel S, Ingle SB, Dhillon S, et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis 2013; 19: 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magro F, Dias CC, Portela F, et al. Development and validation of risk matrices concerning ulcerative colitis outcomes Bayesian network analysis. J Crohns Colitis. Epub ahead of print 17 October 2018. DOI: 10.1093/ecco-jcc/jjy168. [DOI] [PubMed] [Google Scholar]
- 28.Dias CC, Rodrigues PPP, da Costa-Pereira A, et al. Clinical predictors of colectomy in patients with ulcerative colitis: Systematic review and meta-analysis of cohort studies. J Crohns Colitis 2015; 9: 156–163. [DOI] [PubMed] [Google Scholar]
- 29.Al-Darmaki A, Hubbard J, Seow CH, et al. Clinical predictors of the risk of early colectomy in ulcerative colitis: A population-based study. Inflamm Bowel Dis 2017; 23(8): 1272–1277. [DOI] [PubMed] [Google Scholar]