Abstract
Background
The purpose of the present meta-analysis was to provide evident data about use of Apparent Diffusion Coefficient (ADC) values for distinguishing malignant and benign breast lesions.
Methods
MEDLINE library and SCOPUS database were screened for associations between ADC and malignancy/benignancy of breast lesions up to December 2018. Overall, 123 items were identified. The following data were extracted from the literature: authors, year of publication, study design, number of patients/lesions, lesion type, mean value and standard deviation of ADC, measure method, b values, and Tesla strength.
The methodological quality of the 123 studies was checked according to the QUADAS-2 instrument. The meta-analysis was undertaken by using RevMan 5.3 software. DerSimonian and Laird random-effects models with inverse-variance weights were used without any further correction to account for the heterogeneity between the studies. Mean ADC values including 95% confidence intervals were calculated separately for benign and malign lesions.
Results
The acquired 123 studies comprised 13,847 breast lesions. Malignant lesions were diagnosed in 10,622 cases (76.7%) and benign lesions in 3225 cases (23.3%). The mean ADC value of the malignant lesions was 1.03 × 10− 3 mm2/s and the mean value of the benign lesions was 1.5 × 10− 3 mm2/s. The calculated ADC values of benign lesions were over the value of 1.00 × 10− 3 mm2/s. This result was independent on Tesla strength, choice of b values, and measure methods (whole lesion measure vs estimation of ADC in a single area).
Conclusion
An ADC threshold of 1.00 × 10− 3 mm2/s can be recommended for distinguishing breast cancers from benign lesions.
Keywords: Breast cancer, ADC, MRI
Background
Magnetic resonance imaging (MRI) plays an essential diagnostic role in breast cancer (BC) [1, 2]. MRI has been established as the most sensitive diagnostic modality in breast imaging [1–3]. Furthermore, MRI can also predict response to treatment in BC [4]. However, it has a high sensitivity but low specificity [5]. Therefore, MRI can often not distinguish malignant and benign breast lesions. Numerous studies reported that diffusion-weighted imaging (DWI) has a great diagnostic potential and can better characterize breast lesions than conventional MRI [6–8]. DWI is a magnetic resonance imaging (MRI) technique based on measure of water diffusion in tissues [9]. Furthermore, restriction of water diffusion can be quantified by apparent diffusion coefficient (ADC) [9, 10]. It has been shown that malignant tumors have lower values in comparison to benign lesions [7]. In addition, according to the literature, ADC is associated with several histopathological features, such as cell count and expression of proliferation markers, in different tumors [11, 12].
However, use of ADC for discrimination BC and benign breast lesions is difficult because of several problems. Firstly, most reports regarding ADC in several breast cancers and benign breast lesions investigated relatively small patients/lesions samples. Secondly, the studies had different proportions of malignant and benign lesions. Thirdly and most importantly, the reported ADC threshold values and as well specificity, sensitivity, and accuracy values ranged significantly between studies. For example, in the study of Aribal et al., 129 patients with 138 lesions (benign n = 63; malignant n = 75) were enrolled [13]. The authors reported the optimal ADC cut-off as 1.118 × 10− 3 mm2/s with sensitivity and specificity 90.67, and 84.13% respectively [13]. In a study by Arponen et al., which investigated 112 patients (23 benign and 114 malignant lesions), the ADC threshold was 0.87 × 10− 3 mm2/s with 95.7% sensitivity, 89.5% specificity and overall accuracy of 89.8% [14]. Cakir et al. reported in their study with 52 women and 55 breast lesions (30 malignant, 25 benign) an optimal ADC threshold as ≤1.23 × 10− 3 mm2/s (sensitivity = 92.85%, specificity = 54.54%, positive predictive value = 72.22%, negative predictive value = 85.71%, and accuracy = 0.82) [15]. Finally, different MRI scanners, Tesla strengths and b values were used in the reported studies, which are known to have a strong influence in ADC measurements. These facts question the possibility to use the reported ADC thresholds in clinical practice.
To overcome these mentioned shortcomings, the purpose of the present meta-analysis was to provide evident data about use of ADC values for distinguishing malignant and benign breast lesions.
Methods
Data acquisition and proving
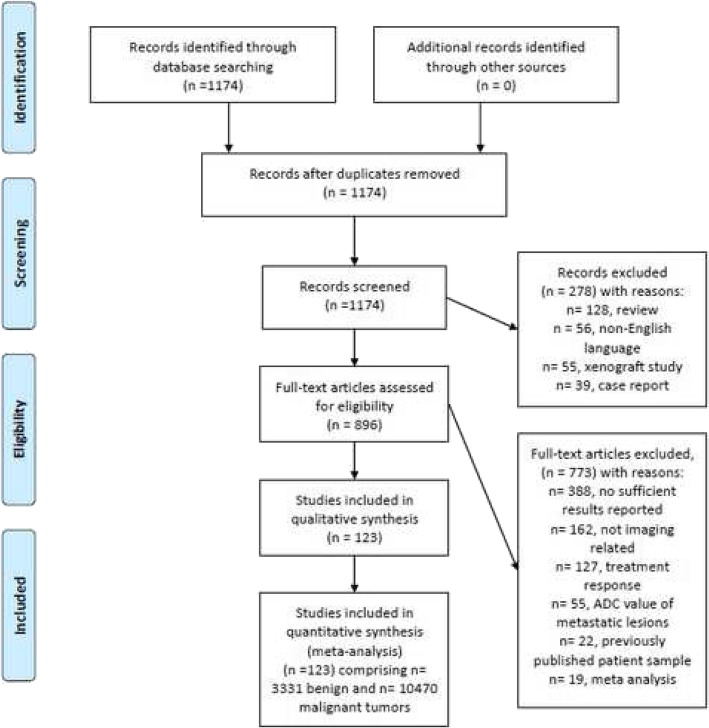
Figure 1 shows the strategy of data acquisition. MEDLINE library and SCOPUS database were screened for associations between ADC and malignancy/benignancy of breast lesions up to December 2018. The following search terms/combinations were as follows:
Fig. 1.
PRISMA flow chart of the data acquisition
“DWI or diffusion weighted imaging or diffusion-weighted imaging or ADC or apparent diffusion coefficient AND breast cancer OR breast carcinoma OR mammary cancer OR breast neoplasm OR breast tumor”. Secondary references were also manually checked and recruited. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) was used for the research [16].
Overall, the primary search identified 1174 records. The abstracts of the items were checked. Inclusion criteria for this work were as follows:
Data regarding ADC derived from diffusion weighted imaging (DWI);
Available mean and standard deviation values of ADC;
Original studies investigated humans;
English language.
Overall, 127 items met the inclusion criteria. Other 1017 records were excluded from the analysis. Exclusion criteria were as follows:
studies unrelated to the research subjects;
studies with incomplete data;
non-English language;
duplicate publications;
experimental animals and in vitro studies;
review, meta-analysis and case report articles;
The following data were extracted from the literature: authors, year of publication, study design, number of patients/lesions, lesion type, mean value and standard deviation of ADC, and Tesla strength.
Meta-analysis
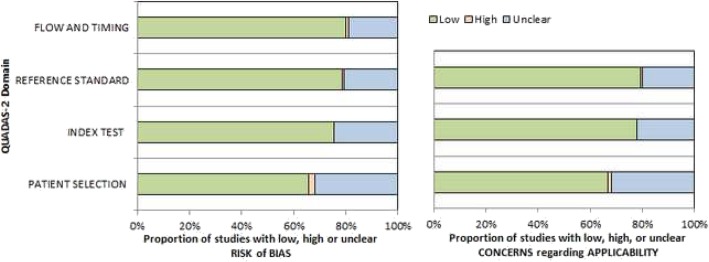
On the first step, the methodological quality of the 123 studies was checked according to the Quality Assessment of Diagnostic Studies (QUADAS-2) instrument [17] independently by two observers (A.S. and H.J.M.). The results of QUADAS-2 assessment are shown in Fig. 2. The quality of most studies showed an overall low risk of bias.
Fig. 2.
QUADAS-2 quality assessment of the included studies
On the second step, the reported ADC values (mean and standard deviation) were acquired from the papers.
Thirdly, the meta-analysis was undertaken by using RevMan 5.3 [RevMan 2014. The Cochrane Collaboration Review Manager Version 5.3.]. Heterogeneity was calculated by means of the inconsistency index I2 [18, 19]. In a subgroup analysis, studies were stratified by tumor type. In addition, DerSimonian and Laird random-effects models with inverse-variance weights were used without any further correction [20] to account for the heterogeneity between the studies (Fig. 3). Mean ADC values including 95% confidence intervals were calculated separately for benign and malign lesions.
Fig. 3.
Funnel plot of the publication bias
Results
Of the included 123 studies, 101 (82.1%) were retrospective and 22 (17.9%) prospective (Table 1). The studies represented almost all continents and originated from Asia (n = 77, 62.6%), Europe (n = 23, 18.7%), North America (n = 19, 15.5%), South America (n = 3, 2.4%), and Africa (n = 1, 0.8%). Different 1.5 T scanners were used in 53 (43.1%) studies, 3 T scanners in 63 reports (51.2%), and in 7 studies (5.7%) both 1.5 and 3 T scanners were used. Overall, 68 studies (55.3%) were performed/reported in the years 2015–2018, 46 studies (37.4%) in the years 2010–2014, and 9 studies (7.3%) in the years 2000–2009.
Table 1.
Studies inclujded into the meta-analysis
| Author, years [Ref.]. | Malignant lesions, n | benign lesions, n | Study design | Tesla strength |
|---|---|---|---|---|
| Akin et al., 2016 [21] | 89 | 92 | retrospective | 3 |
| An et al., 2017 [22] | 112 | 32 | prospective | 3 |
| Arponen et al., 2015 [14] | 114 | 23 | retrospective | 3 |
| Arponen et al., 2018 [23] | 25 | 7 | retrospective | 3 |
| Baba et al., 2014 [24] | 70 | 13 | retrospective | 1.5 |
| Baltzer et al., 2010 [25] | 54 | 27 | retrospective | 1.5 |
| Belli et al., 2015 [26] | 289 | retrospective | 1.5 | |
| Belli et al., 2010 [27] | 100 | 26 | retrospective | 1.5 |
| Bickel et al., 2015 [28] | 176 | retrospective | 3 | |
| Bogner et al., 2009 [29] | 24 | 17 | retrospective | 3 |
| Bokacheva et al., 2014 [30] | 26 | 14 | retrospective | 3 |
| Çabuk et al., 2015 [31] | 22 | 41 | retrospective | 1.5 |
| Cai et al., 2014 [32] | 149 | 85 | retrospective | 1.5 |
| Caivano et al., 2015 [33] | 67 | 43 | retrospective | 3 |
| Cakir et al., 2013 [15] | 30 | 25 | retrospective | 3 |
| Chen et al., 2012 [34] | 39 | 18 | retrospective | 1.5 |
| Chen et al., 2018 [35] | 72 | 44 | prospective | 3 |
| Cheng et al., 2013 [36] | 128 | 60 | retrospective | 1.5 |
| Cho et al., 2016 [37] | 50 | 12 | retrospective | 3 |
| Cho et al., 2015 [38] | 38 | retrospective | 3 | |
| Choi et al., 2017 [39] | 34 | retrospective | 3 and 1.5 | |
| Choi et al., 2018 [40] | 78 | prospective | 3 | |
| Choi et al., 2012 [41] | 335 | retrospective | 1.5 | |
| Choi et al., 2017 [42] | 221 | retrospective | 3 | |
| Cipolla et al., 2014 [43] | 106 | retrospective | 3 | |
| Costantini et al., 2012 [44] | 225 | retrospective | 1.5 | |
| Costantini et al., 2010 [45] | 162 | prospective | 1.5 | |
| de Almeida et al., 2017 [46] | 44 | 37 | retrospective | 1.5 |
| Durando et al., 2016 [47] | 126 | retrospective | 3 | |
| Eghtedari et al., 2016 [48] | 33 | 18 | retrospective | 3 and 1.5 |
| Ertas et al., 2016 [49] | 85 | 85 | retrospective | 3 |
| Ertas et al., 2018 [50] | 85 | 88 | retrospective | 3 |
| Fan et al., 2018 [51] | 126 | retrospective | 3 | |
| Fan et al., 2018 [52] | 68 | 21 | retrospective | 3 |
| Fan et al., 2017 [53] | 82 | retrospective | 3 | |
| Fanariotis et al., 2018 [54] | 59 | 41 | retrospective | 3 |
| Fornasa et al., 2011 [55] | 35 | 43 | retrospective | 1.5 |
| Gity et al., 2018 [56] | 50 | 48 | prospective | 1.5 |
| Guatelli et al., 2017 [57] | 161 | 91 | retrospective | 1.5 |
| Hering et al., 2016 [58] | 25 | 31 | retrospective | 1.5 |
| Hirano et al., 2012 [59] | 48 | 27 | retrospective | 3 |
| Horvat et al., 2018 [60] | 218 | 130 | retrospective | 3 |
| Hu et al., 2018 [61] | 52 | 36 | retrospective | 3 |
| Huang et al., 2018 [62] | 50 | 26 | prospective | 3 |
| Iima et al., 2011 [63] | 25 | retrospective | 1.5 | |
| Imamura et al., 2010 [64] | 16 | 11 | retrospective | 1.5 |
| Inoue et al., 2011 [65] | 91 | 15 | retrospective | 1.5 |
| Janka et al., 2014 [66] | 59 | 20 | retrospective | 1.5 |
| Jeh et al., 2011 [67] | 155 | retrospective | 3 and 1.5 | |
| Jiang et al., 2018 [68] | 171 | 104 | retrospective | 1.5 |
| Jiang et al., 2014 [69] | 64 | retrospective | 1.5 | |
| Jin et al., 2010 [70] | 40 | 20 | retrospective | 1.5 |
| Kanao et al., 2018 [71] | 79 | 83 | retrospective | 3 and 1.5 |
| Kawashima et al., 2017 [72] | 137 | retrospective | 3 | |
| Ei Khouli et al., 2010 [73] | 101 | 33 | retrospective | 3 |
| Kim et al., 2019 [74] | 93 | retrospective | 3 | |
| Kim et al., 2018 [75] | 121 | 48 | retrospective | 3 |
| Kim et al., 2018 [76] | 81 | retrospective | 3 | |
| Kim et al., 2009 [77] | 60 | retrospective | 1.5 | |
| Kitajima et al., 2018 [78] | 67 | retrospective | 3 | |
| Kitajima et al., 2016 [79] | 216 | retrospective | 3 | |
| Köremezli Keskin et al., 2018 [80] | 59 | retrospective | 1.5 | |
| Kul et al., 2018 [81] | 143 | 70 | retrospective | 1.5 |
| Kuroki et al., 2004 [82] | 55 | 5 | retrospective | 1.5 |
| Lee et al., 2016 [83] | 128 | retrospective | 3 | |
| Lee et al., 2016 [84] | 52 | retrospective | 3 | |
| Li et al., 2015 [85] | 55 | retrospective | 3 | |
| Liu et al., 2017 [86] | 48 | 47 | retrospective | 3 |
| Liu et al., 2015 [87] | 176 | retrospective | 3 | |
| Lo et al., 2009 [88] | 20 | 11 | prospective | 3 |
| Matsubayashi et al., 2010 [89] | 26 | retrospective | 1.5 | |
| Min et al., 2015 [90] | 29 | 20 | retrospective | 1.5 |
| Montemezzi et al., 2018 [91] | 453 | prospective | 3 | |
| Mori et al., 2013 [92] | 51 | retrospective | 3 | |
| Nakajo et al., 2010 [93] | 51 | retrospective | 1.5 | |
| Nogueira et al., 2015 [94] | 28 | 30 | prospective | 3 |
| Nogueira et al., 2014 [95] | 89 | 68 | prospective | 3 |
| Ochi et al., 2013 [96] | 59 | 45 | retrospective | 1.5 |
| Onishi et al., 2014 [97] | 17 | retrospective | 3 and 1.5 | |
| Ouyang et al., 2014 [98] | 23 | 16 | retrospective | 3 |
| Park et al., 2017 [99] | 201 | retrospective | 3 | |
| Park et al., 2016 [100] | 71 | prospective | 3 | |
| Park et al., 2007 [101] | 50 | retrospective | 1.5 | |
| Park et al., 2015 [102] | 110 | retrospective | 3 | |
| Parsian et al., 2012 [103] | 175 | retrospective | 1.5 | |
| Parsian et al., 2016 [104] | 26 | retrospective | 1.5 | |
| Partridge et al., 2018 [105] | 242 | prospective | 3 and 1.5 | |
| Partridge et al., 2011 [106] | 27 | 73 | retrospective | 1.5 |
| Partridge et al., 2010 [107] | 29 | 87 | retrospective | 1.5 |
| Partridge et al., 2010 [108] | 21 | 91 | retrospective | 1.5 |
| Pereira et al., 2009 [109] | 26 | 26 | prospective | 1.5 |
| Petralia et al., 2011 [110] | 28 | prospective | 1.5 | |
| Rahbar et al., 2011 [111] | 74 | retrospective | 1.5 | |
| Rahbar et al., 2012 [112] | 36 | retrospective | 1.5 | |
| Ramírez-Galván et al., 2015 [113] | 15 | 21 | prospective | 1.5 |
| Razek et al., 2010 [114] | 66 | prospective | 1.5 | |
| Roknsharifi et al., 2018 [115] | 97 | 59 | retrospective | 1.5 |
| Rubesova et al., 2006 [116] | 65 | 25 | retrospective | 1.5 |
| Sahin et al., 2013 [117] | 35 | 16 | retrospective | 1.5 |
| Satake et al., 2011 [118] | 88 | 27 | retrospective | 3 |
| Sharma et al., 2016 [119] | 259 | 67 | prospective | 1.5 |
| Shen et al., 2018 [120] | 71 | retrospective | 3 | |
| Song et al., 2019 [121] | 85 | retrospective | 3 | |
| Song et al., 2017 [122] | 106 | 25 | prospective | 3 |
| Sonmez et al., 2011 [123] | 25 | 20 | retrospective | 1.5 |
| Spick et al., 2016 [124] | 31 | 24 | prospective | 3 |
| Spick et al., 2016 [125] | 20 | 84 | retrospective | 1.5 |
| Suo et al., 2019 [126] | 134 | retrospective | 3 | |
| Tang et al., 2018 [127] | 54 | 32 | retrospective | 3 |
| Teruel et al., 2016 [128] | 34 | 27 | prospective | 3 |
| Teruel et al., 2016 [129] | 38 | 34 | prospective | 3 |
| Thakur et al., 2018 [130] | 31 | retrospective | 3 | |
| Wan et al., 2016 [131] | 74 | 21 | retrospective | 1.5 |
| Wang et al., 2016 [132] | 31 | 20 | retrospective | 3 |
| Woodhams et al., 2009 [133] | 204 | 58 | prospective | 1.5 |
| Xie et al., 2019 [134] | 134 | retrospective | 3 | |
| Yabuuchi et al., 2006 [135] | 19 | retrospective | 1.5 | |
| Yoo et al., 2014 [136] | 106 | 63 | retrospective | 1.5 |
| Youk et al., 2012 [137] | 271 | retrospective | 3 and 1.5 | |
| Zhang et al., 2019 [138] | 136 | 74 | retrospective | 3 |
| Zhao et al., 2018 [139] | 25 | 23 | retrospective | 3 |
| Zhao et al., 2018 [140] | 119 | 22 | retrospective | 3 |
| Zhou et al., 2018 [141] | 33 | 39 | retrospective | 3 |
The acquired 123 studies comprised 13,847 breast lesions. Malignant lesions were diagnosed in 10,622 cases (76.7%) and benign lesions in 3225 cases (23.3%). The mean ADC value of the malignant lesions was 1.03 × 10− 3 mm2/s and the mean value of the benign lesions was 1.5 × 10− 3 mm2/s (Figs. 4 and 5). Figure 6 shows the distribution of ADC values in malignant and benign lesions. The ADC values of the two groups overlapped significantly. However, there were no benign lesions under the ADC value of 1.00 × 10− 3 mm2/s.
Fig. 4.
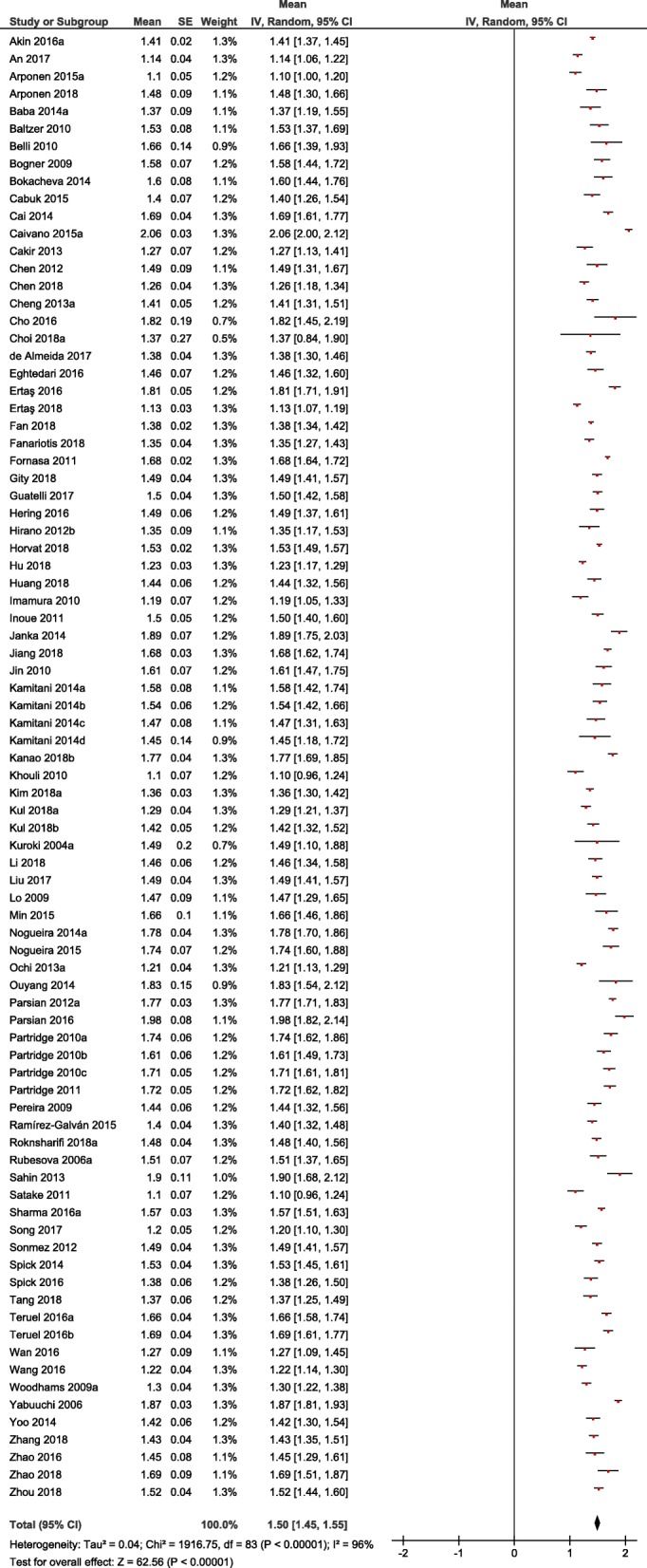
Forrest plots of ADC values reported for benign breast lesions
Fig. 5.
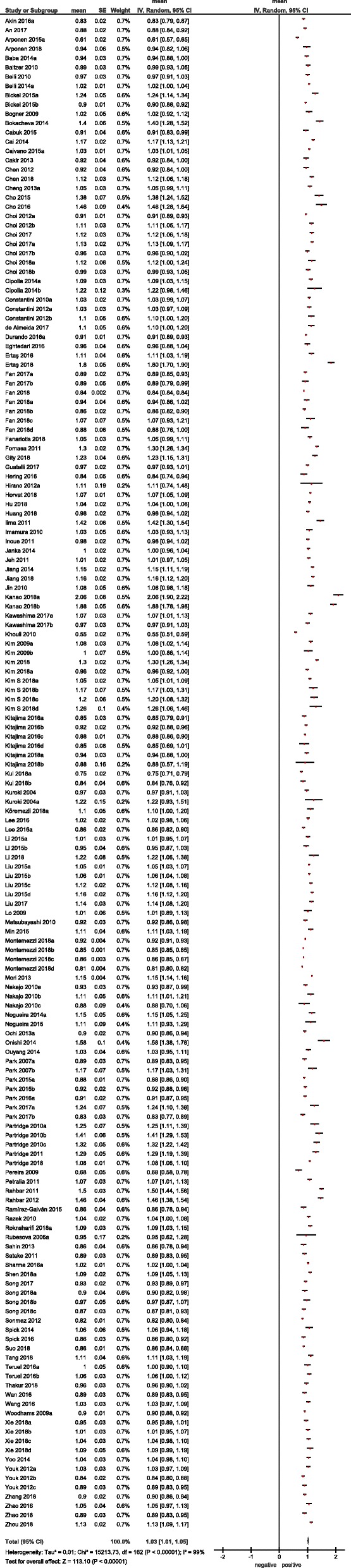
Forrest plots of ADC values reported for malignant breast lesions
Fig. 6.
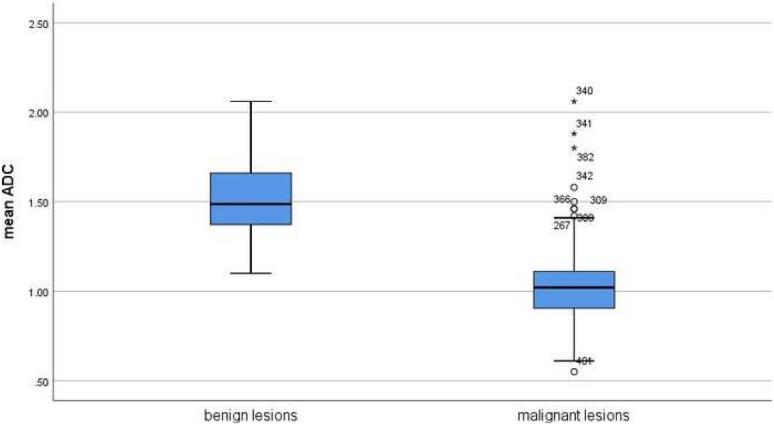
Comparison of ADC values between malignant and benign breast lesions in the overall sample
On the next step ADC values between malignant and benign breast lesions were compared in dependence on Tesla strength. Overall, 5854 lesions were investigated by 1.5 T scanners and 7061 lesions by 3 T scanners. In 932 lesions, the exact information regarding Tesla strength was not given. In the subgroup investigated by 1.5 T scanners, the mean ADC value of the malignant lesions (n = 4093) was 1.05 × 10− 3 mm2/s and the mean value of the benign lesions (n = 1761) was 1.54 × 10− 3 mm2/s (Fig. 7). The ADC values of the benign lesions were upper the ADC value of 1.00 × 10− 3 mm2/s.
Fig. 7.
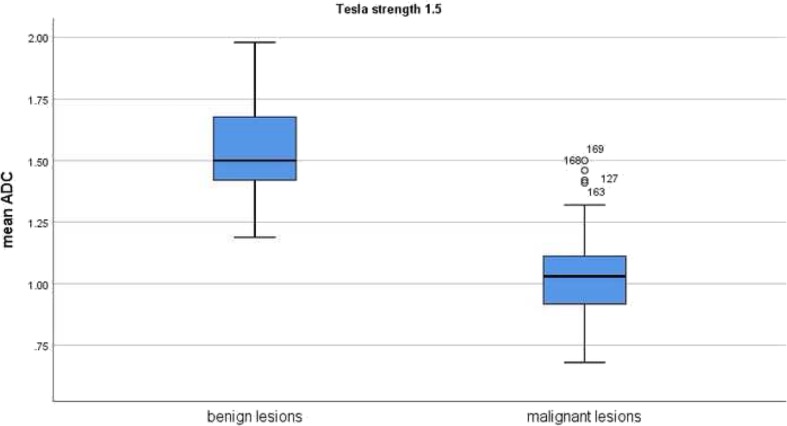
Comparison of ADC values between malignant and benign breast lesions investigated by 1.5 T scanners
In the subgroup investigated by 3 T scanners, the mean ADC values of the malignant lesions (n = 5698) was 1.01 × 10− 3 mm2/s and the mean value of the benign lesions (n = 1363) was 1.46 × 10− 3 mm2/s (Fig. 8). Again in this subgroup, there were no benign lesions under the ADC value of 1.00 × 10− 3 mm2/s.
Fig. 8.
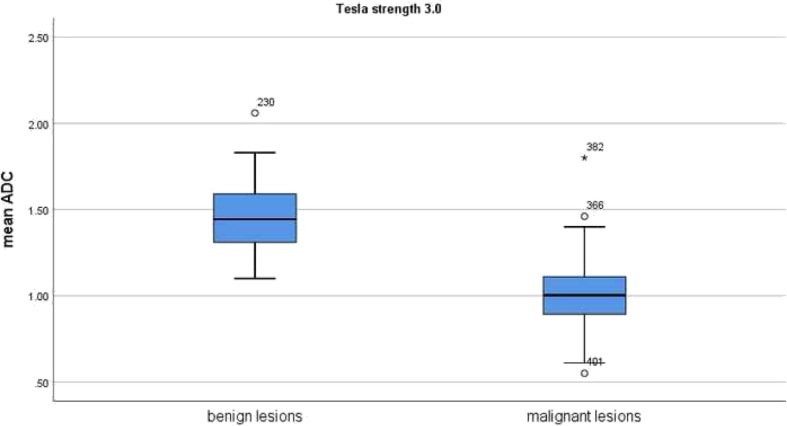
Comparison of ADC values between malignant and benign breast lesions investigated by 3 T scanners
Furthermore, cumulative ADC mean values were calculated in dependence on choice of upper b values. Overall, there were three large subgroups: b600 (426 malignant and 629 benign lesions), b750–850 (4015 malignant and 1230 benign lesions), and b1000 (4396 malignant and 1059 benign lesions). As shown in Fig. 9, the calculated ADC values of benign lesions were over the value 1.00 × 10− 3 mm2/s in every subgroup.
Fig. 9.
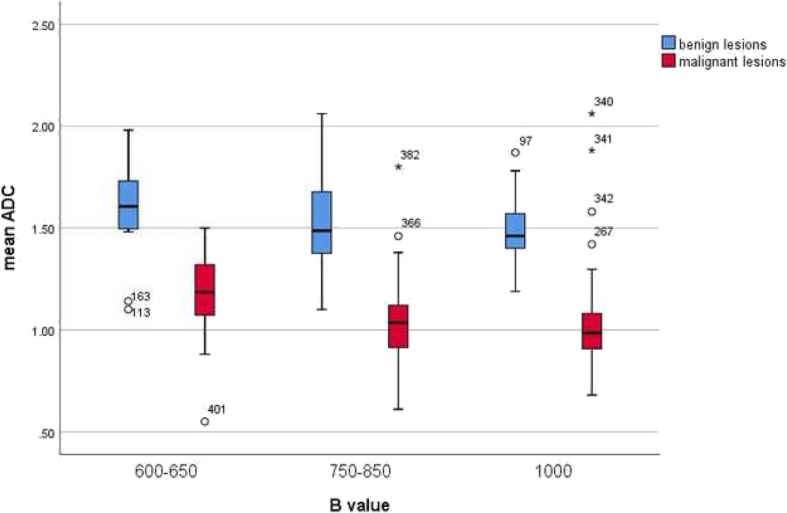
Comparison of ADC values between malignant and benign breast lesions in dependence on the choice of b values
Finally, ADC values of malignant and benign lesions obtained by single measure in an isolated selected area or ROI (region of interest) and whole lesion measure were analyzed. Single ROI measure was performed for 10,882 lesions (8037 malignant and 2845 benign lesions) and whole lesion analysis was used in 2442 cases (1996 malignant and 446 benign lesions). Also in this subgroup, the ADC values of the benign lesions were above the ADC value of 1.00 × 10− 3 mm2/s (Fig. 10).
Fig. 10.
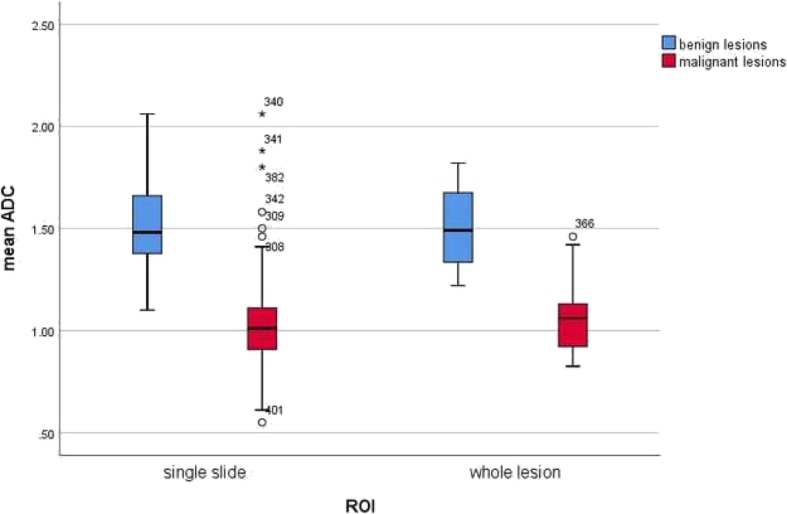
Comparison of ADC values between malignant and benign breast lesions in dependence on measure methods
Discussion
The present analysis investigated ADC values in benign and malignant breast lesions in the largest cohort to date. It addresses a key question as to whether or not imaging parameters, in particular ADC can reflect histopathology of breast lesions. If so, then ADC can be used as a validated imaging biomarker in breast diagnostics. The possibility to stratify breast lesions on imaging is very important and can in particular avoid unnecessary biopsies. As shown in our analysis, previously, numerous studies investigated this question. Interestingly, most studies were reported in the years 2015–2018, which underlines the importance and actuality of the investigated clinical problem. However, as mentioned above, their results were inconsistent. There was no given threshold of an ADC value, which could be used in a clinical setting. Most reports indicated that malignant lesions have lower ADC values than benign findings but there was a broad spectrum of ADC threshold values to discriminate benign and malignant breast lesions. Furthermore, the published results were based on analyses of small numbers of lesions and, therefore, cannot be apply as evident. This limited the possibility to use ADC as an effective diagnostic tool in breast imaging.
Many causes can be responsible for the controversial data. There are no general recommendations regarding use of DWI in breast MRI i.e. Tesla strengths, choice of b values etc. It is known that all the technical parameters can influence DWI and ADC values [142]. Therefore, the reported data cannot apply for every situation. For example, ADC threshold values obtained on 1.5 T scanners cannot be transferred one-to-one to lesions on 3 T.
Furthermore, previous reports had different proportions of benign and malignant lesions comprising various entities. It is well known that some benign breast lesions like abscesses have very low ADC values [143] and some breast cancers, such as mucinous carcinomas, show high ADC values [97, 144]. Furthermore, it has been also shown that invasive ductal and lobular carcinomas had statistically significant lower ADC values in comparison to ductal carcinoma in situ [145]. In addition, also carcinomas with different hormone receptor statuses demonstrate different ADC values [115, 119]. Therefore, the exact proportion of analyzed breast lesions is very important. This suggests also that analyses of ADC values between malignant and benign breast lesions should include all possible lesions. All the facts can explain controversial results of the previous studies but cannot help in a real clinical situation on a patient level basis.
Recently, a meta-analysis about several DWI techniques like diffusion-weighted imaging, diffusion tensor imaging (DTI), and intravoxel incoherent motion (IVIM) in breast imaging was published [146]. It was reported that these techniques were able to discriminate between malignant and benign lesions with a high sensitivity and specificity [146]. However, the authors included only studies with provided sensitivity/specificity data. Furthermore, no threshold values were calculated for discriminating malignant and benign breast lesions. Therefore, no recommendations regarding practical use of DWI in clinical setting could be given.
The present analysis included all published data about DWI findings/ADC values of different breast lesions and, therefore, in contrast to the previous reports, did not have selection bias. It showed that the mean values of benign breast lesions were no lower than 1.00 × 10− 3 mm2/s. Therefore, this value can be used for distinguishing BC from benign findings. Furthermore, this result is independent from Tesla strength, measure methods and from the choice of b values. This fact is very important and suggests that this cut-off can be used in every clinical situation.
We could not find a further threshold in the upper area of ADC values because malignant and benign lesions overlapped significantly. However, most malignant lesions have ADC values under 2.0 × 10− 3 mm2/s. As shown, no real thresholds can be found in the area between 1.00 and 2.00 × 10− 3 mm2/s for discrimination malignant and benign breast lesions.
There are some inherent limitations of the present study to address. Firstly, the meta- analysis is based upon published results in the literature. There might be a certain publication bias because there is a trend to report positive or significant results; whereas studies with insignificant or negative results are often rejected or are not submitted. Secondly, there is the restriction to published papers in English language. Approximately 50 studies could therefore not be included in the present analysis. Thirdly, the study investigated the widely used DWI technique using 2 b-values. However, more advanced MRI sequences, such as intravoxel-incoherent motion and diffusion-kurtosis imaging have been developed, which might show a better accuracy in discriminating benign from malignant tumors. Yet, there are few studies using these sequences and thus no comprehensive analysis can be made.
Conclusion
An ADC threshold of 1.0 × 10− 3 mm2/s can be recommended for distinguishing breast cancers from benign lesions. This result is independent on Tesla strength, choice of b values, and measure methods.
Acknowledgements
None.
Abbreviations
- ADC
Apparent diffusion coefficient
- BC
Breast cancer
- MRI
Magnetic resonance imaging
Authors’ contributions
AS, HJM, AW made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; HJM, AW been involved in drafting the manuscript or revising it critically for important intellectual content; HJM, AW given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content; and AS, HJM, AW agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not Applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alexey Surov, Hans Jonas Meyer and Andreas Wienke contributed equally to this work.
Contributor Information
Alexey Surov, Email: Alexey.Surov@medizin.uni-leipzig.de.
Hans Jonas Meyer, Email: Hans-Jonas.Meyer@medizin.uni-leipzig.de.
Andreas Wienke, Email: andreas.wienke@uk-halle.de.
References
- 1.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European society of breast imaging. Eur Radiol. 2008;18(7):1307–1318. doi: 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004;292(22):2735–2742. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 3.Rahbar H, Partridge SC. Multiparametric MR imaging of breast cancer. Magn Reson Imaging Clin North Am. 2016;24(1):223–238. doi: 10.1016/j.mric.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen R, Jensen LR, Rydland J, et al. Predicting survival and early clinical response to primary chemotherapy for patients with locally advanced breast cancer using DCE-MRI. J Magn Reson Imaging. 2009;29(6):1300–1307. doi: 10.1002/jmri.21778. [DOI] [PubMed] [Google Scholar]
- 5.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26(19):3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Li WL, Zhang YL, et al. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693. doi: 10.1186/1471-2407-10-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altay C, Balci P, Altay S, et al. Diffusion-weighted MR imaging: role in the differential diagnosis of breast lesions. JBR-BTR. 2014;97(4):211–216. doi: 10.5334/jbr-btr.80. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Tang M, Min Z, et al. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol. 2016;57(6):651–660. doi: 10.1177/0284185115597265. [DOI] [PubMed] [Google Scholar]
- 9.Fornasa F. Diffusion-weighted magnetic resonance imaging: what makes water run fast or slow? J Clin Imaging Sci. 2011;1:27. doi: 10.4103/2156-7514.81294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozgeyik Z, Onur MR, Poyraz AK. The role of diffusion weighted magnetic resonance imaging in oncologic settings. Quant Imaging Med Surg. 2013;3(5):269–267. doi: 10.3978/j.issn.2223-4292.2013.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surov A, Meyer HJ, Wienke A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a Meta-analysis. Oncotarget. 2017;8(35):59492–59499. doi: 10.18632/oncotarget.17752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surov A, Meyer HJ, Wienke A. Correlation between apparent diffusion coefficient (ADC) and KI 67 in different tumors: a Meta-analysis. Part 1: ADCmean. Oncotarget. 2017;8(43):75434–75444. doi: 10.18632/oncotarget.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aribal E, Asadov R, Ramazan A, et al. Multiparametric breast MRI with 3T: Effectivity of combination of contrast enhanced MRI, DWI and 1H single voxel spectroscopy in differentiation of breast tumors. Eur J Radiol. 2016;85(5):979–986. doi: 10.1016/j.ejrad.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Arponen O, Sudah M, Masarwah A, et al. Diffusion-Weighted Imaging in 3.0 Tesla Breast MRI: Diagnostic Performance and Tumor Characterization Using Small Subregions vs. Whole Tumor Regions of Interest. PLoS One. 2015;10(10):e0138702. doi: 10.1371/journal.pone.0138702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cakir O, Arslan A, Inan N, et al. Comparison of the diagnostic performances of diffusion parameters in diffusion weighted imaging and diffusion tensor imaging of breast lesions. Eur J Radiol. 2013;82(12):e801–6. [DOI] [PubMed]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149(12):889–897. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Akın Y, Uğurlu MÜ, Kaya H, Arıbal E. Diagnostic value of diffusion-weighted imaging and apparent diffusion coefficient values in the differentiation of breast lesions, Histpathologic subgroups and Correlatıon with Prognostıc factors using 3.0 tesla MR. J Breast Health. 2016;12(3):123–132. doi: 10.5152/tjbh.2016.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An YY, Kim SH, Kang BJ. Differentiation of malignant and benign breast lesions: Added value of the qualitative analysis of breast lesions on diffusion-weighted imaging (DWI) using readout-segmented echo-planar imaging at 3.0 T. PLoS One. 2017;12(3):e0174681. doi: 10.1371/journal.pone.0174681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arponen O, Sudah M, Sutela A, et al. Gadoterate meglumine decreases ADC values of breast lesions depending on the b value combination. Sci Rep. 2018;8(1):87. doi: 10.1038/s41598-017-18035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba S, Isoda T, Maruoka Y, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55(5):736–742. doi: 10.2967/jnumed.113.129395. [DOI] [PubMed] [Google Scholar]
- 25.Baltzer PA, Benndorf M, Dietzel M, et al. Sensitivity and specificity of unenhanced MR mammography (DWI combined with T2-weighted TSE imaging, ueMRM) for the differentiation of mass lesions. Eur Radiol. 2010;20(5):1101–1110. doi: 10.1007/s00330-009-1654-5. [DOI] [PubMed] [Google Scholar]
- 26.Belli P, Costantini M, Bufi E, et al. Diffusion magnetic resonance imaging in breast cancer characterisation: correlations between the apparent diffusion coefficient and major prognostic factors. Radiol Med. 2015;120(3):268–276. doi: 10.1007/s11547-014-0442-8. [DOI] [PubMed] [Google Scholar]
- 27.Belli P, Costantini M, Bufi E, et al. Diffusion-weighted imaging in breast lesion evaluation. Radiol Med. 2010;115(1):51–69. doi: 10.1007/s11547-009-0430-6. [DOI] [PubMed] [Google Scholar]
- 28.Bickel H, Pinker-Domenig K, Bogner W, et al. Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Investig Radiol. 2015;50(2):95–100. doi: 10.1097/RLI.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 29.Bogner W, Gruber S, Pinker K, et al. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology. 2009;253(2):341–351. doi: 10.1148/radiol.2532081718. [DOI] [PubMed] [Google Scholar]
- 30.Bokacheva L, Kaplan JB, Giri DD, et al. Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. J Magn Reson Imaging. 2014;40(4):813–823. doi: 10.1002/jmri.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Çabuk G, Nass Duce M, Özgür A, et al. The diagnostic value of diffusion-weighted imaging and the apparent diffusion coefficient values in the differentiation of benign and malignant breast lesions. J Med Imaging Radiat Oncol. 2015;59(2):141–148. doi: 10.1111/1754-9485.12273. [DOI] [PubMed] [Google Scholar]
- 32.Cai H, Peng Y, Ou C, et al. Diagnosis of breast masses from dynamic contrast-enhanced and diffusion-weighted MR: a machine learning approach. PLoS One. 2014;9(1):e87387. doi: 10.1371/journal.pone.0087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caivano R, Villonio A, D’Antuono F, et al. Diffusion weighted imaging and apparent diffusion coefficient in 3 tesla magnetic resonance imaging of breast lesions. Cancer Investig. 2015;33(5):159–164. doi: 10.3109/07357907.2015.1019674. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, He XJ, Jin R, et al. Conspicuity of breast lesions at different b values on diffusion-weighted imaging. BMC Cancer. 2012;12:334. doi: 10.1186/1471-2407-12-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Wu B, Liu H, et al. Feasibility study of dual parametric 2D histogram analysis of breast lesions with dynamic contrast-enhanced and diffusion-weighted MRI. J Transl Med. 2018;16(1):325. doi: 10.1186/s12967-018-1698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng L, Bai Y, Zhang J, et al. Optimization of apparent diffusion coefficient measured by diffusion-weighted MRI for diagnosis of breast lesions presenting as mass and non-mass-like enhancement. Tumour Biol. 2013;34(3):1537–1545. doi: 10.1007/s13277-013-0682-6. [DOI] [PubMed] [Google Scholar]
- 37.Cho GY, Moy L, Kim SG, et al. Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur Radiol. 2016;26(8):2547–2558. doi: 10.1007/s00330-015-4087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho GY, Moy L, Kim SG, et al. Comparison of contrast enhancement and diffusion-weighted magnetic resonance imaging in healthy and cancerous breast tissue. Eur J Radiol. 2015;84(10):1888–1893. doi: 10.1016/j.ejrad.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Choi BB, Kim SH, Park CS, Jung NY. Correlation of prognostic factors of invasive lobular carcinoma with ADC value of DWI and SUVMax of FDG-PET. Chonnam Med J. 2017;53(2):133–139. doi: 10.4068/cmj.2017.53.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi JH, Lim I, Noh WC, et al. Prediction of tumor differentiation using sequential PET/CT and MRI in patients with breast cancer. Ann Nucl Med. 2018;32(6):389–397. doi: 10.1007/s12149-018-1259-7. [DOI] [PubMed] [Google Scholar]
- 41.Choi SY, Chang YW, Park HJ, et al. Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br J Radiol. 2012;85(1016):e474–e479. doi: 10.1259/bjr/79381464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi Y, Kim SH, Youn IK, et al. Rim sign and histogram analysis of apparent diffusion coefficient values on diffusion-weighted MRI in triple-negative breast cancer: comparison with ER-positive subtype. PLoS One. 2017;12(5):e0177903. doi: 10.1371/journal.pone.0177903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cipolla V, Santucci D, Guerrieri D, et al. Correlation between 3T apparent diffusion coefficient values and grading of invasive breast carcinoma. Eur J Radiol. 2014;83(12):2144–2150. doi: 10.1016/j.ejrad.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Costantini M, Belli P, Distefano D, et al. Magnetic resonance imaging features in triple-negative breast cancer: comparison with luminal and HER2-overexpressing tumors. Clin Breast Cancer. 2012;12(5):331–339. doi: 10.1016/j.clbc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Costantini M, Belli P, Rinaldi P, et al. Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clin Radiol. 2010;65(12):1005–12. doi: 10.1016/j.crad.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 46.de Almeida JRM, Gomes AB, Barros TP, et al. Diffusion-weighted imaging of suspicious (BI-RADS 4) breast lesions: stratification based on histopathology. Radiol Bras. 2017;50(3):154–161. doi: 10.1590/0100-3984.2015.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durando M, Gennaro L, Cho GY, et al. Quantitative apparent diffusion coefficient measurement obtained by 3.0Tesla MRI as a potential noninvasive marker of tumor aggressiveness in breast cancer. Eur J Radiol. 2016;85(9):1651–1658. doi: 10.1016/j.ejrad.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eghtedari M, Ma J, Fox P, et al. Effects of magnetic field strength and b value on the sensitivity and specificity of quantitative breast diffusion-weighted MRI. Quant Imaging Med Surg. 2016;6(4):374–380. doi: 10.21037/qims.2016.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ertas G, Onaygil C, Akin Y, et al. Quantitative differentiation of breast lesions at 3T diffusion-weighted imaging (DWI) using the ratio of distributed diffusion coefficient (DDC) J Magn Reson Imaging. 2016;44(6):1633–1641. doi: 10.1002/jmri.25327. [DOI] [PubMed] [Google Scholar]
- 50.Ertaş G, Onaygil C, Buğdaycı O, Arıbal E. Dual-phase ADC modelling of breast masses in diffusion-weighted imaging: comparison with Histopathologic findings. Eur J Breast Health. 2018;14(2):85–92. doi: 10.5152/ejbh.2018.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan M, He T, Zhang P, et al. Diffusion-weighted imaging features of breast tumours and the surrounding stroma reflect intrinsic heterogeneous characteristics of molecular subtypes in breast cancer. NMR Biomed. 2018;31(2). 10.1002/nbm.3869 [DOI] [PubMed]
- 52.Fan WX, Chen XF, Cheng FY, et al. Retrospective analysis of the utility of multiparametric MRI for differentiating between benign and malignant breast lesions in women in China. Medicine (Baltimore) 2018;97(4):e9666. doi: 10.1097/MD.0000000000009666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan M, He T, Zhang P, et al. Heterogeneity of diffusion-weighted imaging in tumours and the surrounding Stroma for prediction of Ki-67 proliferation status in breast Cancer. Sci Rep. 2017;7(1):2875. doi: 10.1038/s41598-017-03122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fanariotis M, Vassiou K, Tsougos I, Fezoulidis I. Reproducibility of apparent diffusion coefficient measurements evaluated with different workstations. Clin Radiol. 2018;73(2):141–148. doi: 10.1016/j.crad.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Fornasa F, Pinali L, Gasparini A, et al. Diffusion-weighted magnetic resonance imaging in focal breast lesions: analysis of 78 cases with pathological correlation. Radiol Med. 2011;116(2):264–275. doi: 10.1007/s11547-010-0602-4. [DOI] [PubMed] [Google Scholar]
- 56.Gity M, Moradi B, Arami R, et al. Two different methods of region-of-interest placement for differentiation of benign and malignant breast lesions by apparent diffusion coefficient value. Asian Pac J Cancer Prev. 2018;19(10):2765–2770. doi: 10.22034/APJCP.2018.19.10.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guatelli CS, Bitencourt AGV, Osório CABT, et al. Can diffusion-weighted imaging add information in the evaluation of breast lesions considered suspicious on magnetic resonance imaging? Radiol Bras. 2017;50(5):291–298. doi: 10.1590/0100-3984.2016.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hering J, Laun FB, Lederer W, et al. Applicability and discriminative value of a semiautomatic three-dimensional spherical volume for the assessment of the apparent diffusion coefficient in suspicious breast lesions-feasibility study. Clin Imaging. 2016;40(6):1280–1285. doi: 10.1016/j.clinimag.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 59.Hirano M, Satake H, Ishigaki S, et al. Diffusion-weighted imaging of breast masses: comparison of diagnostic performance using various apparent diffusion coefficient parameters. AJR Am J Roentgenol. 2012;198(3):717–722. doi: 10.2214/AJR.11.7093. [DOI] [PubMed] [Google Scholar]
- 60.Horvat JV, Durando M, Milans S, et al. Apparent diffusion coefficient mapping using diffusion-weighted MRI: impact of background parenchymal enhancement, amount of fibroglandular tissue and menopausal status on breast cancer diagnosis. Eur Radiol. 2018;28(6):2516–2524. doi: 10.1007/s00330-017-5202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu B, Xu K, Zhang Z, et al. A radiomic nomogram based on an apparent diffusion coefficient map for differential diagnosis of suspicious breast findings. Chin J Cancer Res. 2018;30(4):432–438. doi: 10.21147/j.issn.1000-9604.2018.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Y, Lin Y, Hu W, et al. Diffusion kurtosis at 3.0T as an in vivo imaging marker for breast Cancer characterization: correlation with prognostic factors. J Magn Reson Imaging. 2019;49(3):845–856. doi: 10.1002/jmri.26249. [DOI] [PubMed] [Google Scholar]
- 63.Iima M, Le Bihan D, Okumura R, et al. Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study. Radiology. 2011;260(2):364–372. doi: 10.1148/radiol.11101892. [DOI] [PubMed] [Google Scholar]
- 64.Imamura T, Isomoto I, Sueyoshi E, et al. Diagnostic performance of ADC for non-mass-like breast lesions on MR imaging. Magn Reson Med Sci. 2010;9(4):217–225. doi: 10.2463/mrms.9.217. [DOI] [PubMed] [Google Scholar]
- 65.Inoue K, Kozawa E, Mizukoshi W, et al. Usefulness of diffusion-weighted imaging of breast tumors: quantitative and visual assessment. Jpn J Radiol. 2011;29(6):429–436. doi: 10.1007/s11604-011-0575-9. [DOI] [PubMed] [Google Scholar]
- 66.Janka R, Hammon M, Geppert C, et al. Diffusion-weighted MR imaging of benign and malignant breast lesions before and after contrast enhancement. Rofo. 2014;186(2):130–135. doi: 10.1055/s-0033-1350298. [DOI] [PubMed] [Google Scholar]
- 67.Jeh SK, Kim SH, Kim HS, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging. 2011;33(1):102–109. doi: 10.1002/jmri.22400. [DOI] [PubMed] [Google Scholar]
- 68.Jiang X, Xie F, Liu L, et al. Discrimination of malignant and benign breast masses using automatic segmentation and features extracted from dynamic contrast-enhanced and diffusion-weighted MRI. Oncol Lett. 2018;16(2):1521–1528. doi: 10.3892/ol.2018.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang XY, Xie QZ, Cao XS, et al. Value of diffusion weighted imaging in the differential diagnosis of benign and malignant breast lesions at 3.0T MRI. Genet Mol Res. 2014;13(3):7773–7779. doi: 10.4238/2014.September.26.15. [DOI] [PubMed] [Google Scholar]
- 70.Jin G, An N, Jacobs MA, Li K. The role of parallel diffusion-weighted imaging and apparent diffusion coefficient (ADC) map values for evaluating breast lesions: preliminary results. Acad Radiol. 2010;17(4):456–463. doi: 10.1016/j.acra.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanao S, Kataoka M, Iima M, et al. Differentiating benign and malignant inflammatory breast lesions: value of T2 weighted and diffusion weighted MR images. Magn Reson Imaging. 2018;50:38–44. doi: 10.1016/j.mri.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Kawashima H, Miyati T, Ohno N, et al. Differentiation between luminal-a and luminal-B breast Cancer using Intravoxel incoherent motion and dynamic contrast-enhanced magnetic resonance imaging. Acad Radiol. 2017;24(12):1575–1581. doi: 10.1016/j.acra.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 73.Ei Khouli RH, Jacobs MA, Mezban SD, et al. Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology. 2010;256(1):64–73. doi: 10.1148/radiol.10091367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JY, Kim JJ, Lee JW, et al. Risk stratification of ductal carcinoma in situ using whole-lesion histogram analysis of the apparent diffusion coefficient. Eur Radiol. 2019;29(2):485–493. doi: 10.1007/s00330-018-5666-x. [DOI] [PubMed] [Google Scholar]
- 75.Kim KW, Kuzmiak CM, Kim YJ, et al. Diagnostic usefulness of combination of diffusion-weighted imaging and T2WI, including apparent diffusion coefficient in breast lesions: assessment of histologic grade. Acad Radiol. 2018;25(5):643–652. doi: 10.1016/j.acra.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 76.Kim SY, Shin J, Kim DH, et al. Correlation between electrical conductivity and apparent diffusion coefficient in breast cancer: effect of necrosis on magnetic resonance imaging. Eur Radiol. 2018;28(8):3204–3214. doi: 10.1007/s00330-017-5291-0. [DOI] [PubMed] [Google Scholar]
- 77.Kim SH, Cha ES, Kim HS, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging. 2009;30(3):615–620. doi: 10.1002/jmri.21884. [DOI] [PubMed] [Google Scholar]
- 78.Kitajima K, Miyoshi Y, Yamano T, et al. Prognostic value of FDG-PET and DWI in breast cancer. Ann Nucl Med. 2018;32(1):44–53. doi: 10.1007/s12149-017-1217-9. [DOI] [PubMed] [Google Scholar]
- 79.Kitajima K, Yamano T, Fukushima K, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol. 2016;85(5):943–949. doi: 10.1016/j.ejrad.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 80.Köremezli Keskin N, Balcı P, Başara Akın I, et al. Detection of the differences in the apparent diffusion coefficient values in different histopathological types of malignant breast lesions and comparison of cellular region/ stroma ratio and histopathological results. Turk J Med Sci. 2018;48(4):817–825. doi: 10.3906/sag-1801-89. [DOI] [PubMed] [Google Scholar]
- 81.Kul S, Metin Y, Kul M, et al. Assessment of breast mass morphology with diffusion-weighted MRI: beyond apparent diffusion coefficient. J Magn Reson Imaging. 2018;48(6):1668–1677. doi: 10.1002/jmri.26175. [DOI] [PubMed] [Google Scholar]
- 82.Kuroki Y, Nasu K, Kuroki S, et al. Diffusion-weighted imaging of breast cancer with the sensitivity encoding technique: analysis of the apparent diffusion coefficient value. Magn Reson Med Sci. 2004;3(2):79–85. doi: 10.2463/mrms.3.79. [DOI] [PubMed] [Google Scholar]
- 83.Lee CW, Wu HK, Lai HW, et al. Preoperative clinicopathologic factors and breast magnetic resonance imaging features can predict ductal carcinoma in situ with invasive components. Eur J Radiol. 2016;85(4):780–789. doi: 10.1016/j.ejrad.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 84.Lee HS, Kim SH, Kang BJ, et al. Perfusion Parameters in Dynamic Contrast-enhanced MRI and Apparent Diffusion Coefficient Value in Diffusion-weighted MRI:: Association with Prognostic Factors in Breast Cancer. Acad Radiol. 2016;23(4):446–456. doi: 10.1016/j.acra.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Li L, Wang K, Sun X, et al. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit. 2015;21:376–382. doi: 10.12659/MSM.892534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu HL, Zong M, Wei H, et al. Preoperative predicting malignancy in breast mass-like lesions: value of adding histogram analysis of apparent diffusion coefficient maps to dynamic contrast-enhanced magnetic resonance imaging for improving confidence level. Br J Radiol. 2017;90(1079):20170394. doi: 10.1259/bjr.20170394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu S, Ren R, Chen Z, et al. Diffusion-weighted imaging in assessing pathological response of tumor in breast cancer subtype to neoadjuvant chemotherapy. J Magn Reson Imaging. 2015;42(3):779–787. doi: 10.1002/jmri.24843. [DOI] [PubMed] [Google Scholar]
- 88.Lo GG, Ai V, Chan JK, et al. Diffusion-weighted magnetic resonance imaging of breast lesions: first experiences at 3 T. J Comput Assist Tomogr. 2009;33(1):63–69. doi: 10.1097/RCT.0b013e318165dc6b. [DOI] [PubMed] [Google Scholar]
- 89.Matsubayashi RN, Fujii T, Yasumori K, et al. Apparent Diffusion Coefficient in Invasive Ductal Breast Carcinoma: Correlation with Detailed Histologic Features and the Enhancement Ratio on Dynamic Contrast-Enhanced MR Images. J Oncol. 2010;2010. 10.1155/2010/821048 [DOI] [PMC free article] [PubMed]
- 90.Min Q, Shao K, Zhai L, et al. Differential diagnosis of benign and malignant breast masses using diffusion-weighted magnetic resonance imaging. World J Surg Oncol. 2015;13:32. doi: 10.1186/s12957-014-0431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Montemezzi S, Camera L, Giri MG, et al. Is there a correlation between 3T multiparametric MRI and molecular subtypes of breast cancer? Eur J Radiol. 2018;108:120–127. doi: 10.1016/j.ejrad.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 92.Mori N, Ota H, Mugikura S, et al. Detection of invasive components in cases of breast ductal carcinoma in situ on biopsy by using apparent diffusion coefficient MR parameters. Eur Radiol. 2013;23(10):2705–2712. doi: 10.1007/s00330-013-2902-2. [DOI] [PubMed] [Google Scholar]
- 93.Nakajo M, Kajiya Y, Kaneko T, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010;37(11):2011–2020. doi: 10.1007/s00259-010-1529-7. [DOI] [PubMed] [Google Scholar]
- 94.Nogueira L, Brandão S, Matos E, et al. Improving malignancy prediction in breast lesions with the combination of apparent diffusion coefficient and dynamic contrast-enhanced kinetic descriptors. Clin Radiol. 2015;70(9):1016–1025. doi: 10.1016/j.crad.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 95.Nogueira L, Brandão S, Matos E, et al. Diffusion-weighted imaging: determination of the best pair of b-values to discriminate breast lesions. Br J Radiol. 2014;87(1039):20130807. doi: 10.1259/bjr.20130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ochi M, Kuroiwa T, Sunami S, et al. Diffusion-weighted imaging (b value = 1500 s/mm(2)) is useful to decrease false-positive breast cancer cases due to fibrocystic changes. Breast Cancer. 2013;20(2):137–144. doi: 10.1007/s12282-011-0319-9. [DOI] [PubMed] [Google Scholar]
- 97.Onishi N, Kanao S, Kataoka M, et al. Apparent diffusion coefficient as a potential surrogate marker for Ki-67 index in mucinous breast carcinoma. J Magn Reson Imaging. 2015;41(3):610–615. doi: 10.1002/jmri.24615. [DOI] [PubMed] [Google Scholar]
- 98.Ouyang Z, Ouyang Y, Zhu M, et al. Diffusion-weighted imaging with fat suppression using short-tau inversion recovery: clinical utility for diagnosis of breast lesions. Clin Radiol. 2014;69(8):e337–e344. doi: 10.1016/j.crad.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 99.Park GE, Kim SH, Kim EJ, et al. Histogram analysis of volume-based apparent diffusion coefficient in breast cancer. Acta Radiol. 2017;58(11):1294–1302. doi: 10.1177/0284185117694507. [DOI] [PubMed] [Google Scholar]
- 100.Park EK, Cho KR, Seo BK, et al. Additional value of diffusion-weighted imaging to evaluate prognostic factors of breast Cancer: correlation with the apparent diffusion coefficient. Iran J Radiol. 2016;13(1):e33133. doi: 10.5812/iranjradiol.33133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park MJ, Cha ES, Kang BJ, et al. The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol. 2007;8(5):390–396. doi: 10.3348/kjr.2007.8.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park SH, Choi HY, Hahn SY. Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 tesla. J Magn Reson Imaging. 2015;41(1):175–182. doi: 10.1002/jmri.24519. [DOI] [PubMed] [Google Scholar]
- 103.Parsian S, Rahbar H, Allison KH, et al. Nonmalignant breast lesions: ADCs of benign and high-risk subtypes assessed as false-positive at dynamic enhanced MR imaging. Radiology. 2012;265(3):696–706. doi: 10.1148/radiol.12112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parsian S, Giannakopoulos NV, Rahbar H, et al. Diffusion-weighted imaging reflects variable cellularity and stromal density present in breast fibroadenomas. Clin Imaging. 2016;40(5):1047–1054. doi: 10.1016/j.clinimag.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Partridge SC, Zhang Z, Newitt DC, et al. Diffusion-weighted MRI findings predict pathologic response in Neoadjuvant treatment of breast Cancer: the ACRIN 6698 Multicenter trial. Radiology. 2018;289(3):618–627. doi: 10.1148/radiol.2018180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Partridge SC, Rahbar H, Murthy R, et al. Improved diagnostic accuracy of breast MRI through combined apparent diffusion coefficients and dynamic contrast-enhanced kinetics. Magn Reson Med. 2011;65(6):1759–1767. doi: 10.1002/mrm.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Partridge SC, Mullins CD, Kurland BF, et al. Apparent diffusion coefficient values for discriminating benign and malignant breast MRI lesions: effects of lesion type and size. AJR Am J Roentgenol. 2010;194(6):1664–1673. doi: 10.2214/AJR.09.3534. [DOI] [PubMed] [Google Scholar]
- 108.Partridge SC, Demartini WB, Kurland BF, et al. Differential diagnosis of mammographically and clinically occult breast lesions on diffusion-weighted MRI. J Magn Reson Imaging. 2010;31(3):562–570. doi: 10.1002/jmri.22078. [DOI] [PubMed] [Google Scholar]
- 109.Pereira FP, Martins G, Figueiredo E, et al. Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different b values. AJR Am J Roentgenol. 2009;193(4):1030–1035. doi: 10.2214/AJR.09.2522. [DOI] [PubMed] [Google Scholar]
- 110.Petralia G, Bonello L, Summers P, et al. Intraobserver and interobserver variability in the calculation of apparent diffusion coefficient (ADC) from diffusion-weighted magnetic resonance imaging (DW-MRI) of breast tumours. Radiol Med. 2011;116(3):466–476. doi: 10.1007/s11547-011-0616-z. [DOI] [PubMed] [Google Scholar]
- 111.Rahbar H, Partridge SC, Eby PR, et al. Characterization of ductal carcinoma in situ on diffusion weighted breast MRI. Eur Radiol. 2011;21(9):2011–2019. doi: 10.1007/s00330-011-2140-4. [DOI] [PubMed] [Google Scholar]
- 112.Rahbar H, Partridge SC, Demartini WB, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology. 2012;263(2):374–382. doi: 10.1148/radiol.12111368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramírez-Galván YA, Cardona-Huerta S, Ibarra-Fombona E, Elizondo-Riojas G. Apparent diffusion coefficient (ADC) value to evaluate BI-RADS 4 breast lesions: correlation with pathological findings. Clin Imaging. 2015;39(1):51–55. doi: 10.1016/j.clinimag.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 114.Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010;23(6):619–623. doi: 10.1002/nbm.1503. [DOI] [PubMed] [Google Scholar]
- 115.Roknsharifi S, Fishman MDC, Agarwal MD, et al. The role of diffusion weighted imaging as supplement to dynamic contrast enhanced breast MRI: Can it help predict malignancy, histologic grade and recurrence? Acad Radiol. 2019;26(7):923–29. [DOI] [PubMed]
- 116.Rubesova E, Grell AS, De Maertelaer V, et al. Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging. 2006;24(2):319–324. doi: 10.1002/jmri.20643. [DOI] [PubMed] [Google Scholar]
- 117.Şahin C, Arıbal E. The role of apparent diffusion coefficient values in the differential diagnosis of breast lesions in diffusion-weighted MRI. Diagn Interv Radiol. 2013;19(6):457–462. doi: 10.5152/dir.2013.12132. [DOI] [PubMed] [Google Scholar]
- 118.Satake H, Nishio A, Ikeda M, et al. Predictive value for malignancy of suspicious breast masses of BI-RADS categories 4 and 5 using ultrasound elastography and MR diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196(1):202–209. doi: 10.2214/AJR.09.4108. [DOI] [PubMed] [Google Scholar]
- 119.Sharma U, Sah RG, Agarwal K, et al. Potential of diffusion-weighted imaging in the characterization of malignant, benign, and healthy breast tissues and molecular subtypes of breast Cancer. Front Oncol. 2016;6:126. doi: 10.3389/fonc.2016.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen L, Zhou G, Tong T, et al. ADC at 3.0 T as a noninvasive biomarker for preoperative prediction of Ki67 expression in invasive ductal carcinoma of breast. Clin Imaging. 2018;52:16–22. doi: 10.1016/j.clinimag.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 121.Song SE, Cho KR, Seo BK, et al. Intravoxel incoherent motion diffusion-weighted MRI of invasive breast cancer: correlation with prognostic factors and kinetic features acquired with computer-aided diagnosis. J Magn Reson Imaging. 2019;49(1):118–130. doi: 10.1002/jmri.26221. [DOI] [PubMed] [Google Scholar]
- 122.Song SE, Park EK, Cho KR, et al. Additional value of diffusion-weighted imaging to evaluate multifocal and multicentric breast cancer detected using pre-operative breast MRI. Eur Radiol. 2017;27(11):4819–4827. doi: 10.1007/s00330-017-4898-5. [DOI] [PubMed] [Google Scholar]
- 123.Sonmez G, Cuce F, Mutlu H, et al. Value of diffusion-weighted MRI in the differentiation of benign and malign breast lesions. Wien Klin Wochenschr. 2011;123(21–22):655–661. doi: 10.1007/s00508-011-0053-5. [DOI] [PubMed] [Google Scholar]
- 124.Spick C, Bickel H, Pinker K, et al. Diffusion-weighted MRI of breast lesions: a prospective clinical investigation of the quantitative imaging biomarker characteristics of reproducibility, repeatability, and diagnostic accuracy. NMR Biomed. 2016;29(10):1445–1453. doi: 10.1002/nbm.3596. [DOI] [PubMed] [Google Scholar]
- 125.Spick C, Pinker-Domenig K, Rudas M, et al. MRI-only lesions: application of diffusion-weighted imaging obviates unnecessary MR-guided breast biopsies. Eur Radiol. 2014;24(6):1204–1210. doi: 10.1007/s00330-014-3153-6. [DOI] [PubMed] [Google Scholar]
- 126.Suo S, Zhang D, Cheng F, et al. Added value of mean and entropy of apparent diffusion coefficient values for evaluating histologic phenotypes of invasive ductal breast cancer with MR imaging. Eur Radiol. 2019;29(3):1425–1434. doi: 10.1007/s00330-018-5667-9. [DOI] [PubMed] [Google Scholar]
- 127.Tang Q, Li Q, Xie D, et al. An apparent diffusion coefficient histogram method versus a traditional 2-dimensional measurement method for identifying non-puerperal mastitis from breast Cancer at 3.0 T. J Comput Assist Tomogr. 2018;42(5):776–783. doi: 10.1097/RCT.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 128.Teruel JR, Goa PE, Sjøbakk TE, et al. A simplified approach to measure the effect of the microvasculature in diffusion-weighted MR imaging applied to breast Tumors: preliminary results. Radiology. 2016;281(2):373–381. doi: 10.1148/radiol.2016151630. [DOI] [PubMed] [Google Scholar]
- 129.Teruel JR, Goa PE, Sjøbakk TE, et al. Diffusion weighted imaging for the differentiation of breast tumors: from apparent diffusion coefficient to high order diffusion tensor imaging. J Magn Reson Imaging. 2016;43(5):1111–1121. doi: 10.1002/jmri.25067. [DOI] [PubMed] [Google Scholar]
- 130.Thakur SB, Durando M, Milans S, et al. Apparent diffusion coefficient in estrogen receptor-positive and lymph node-negative invasive breast cancers at 3.0T DW-MRI: a potential predictor for an oncotype dx test recurrence score. J Magn Reson Imaging. 2018;47(2):401–409. doi: 10.1002/jmri.25796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wan CW, Lee CY, Lui CY, et al. Apparent diffusion coefficient in differentiation between malignant and benign breast masses: does size matter? Clin Radiol. 2016;71(2):170–177. doi: 10.1016/j.crad.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 132.Wang Q, Guo Y, Zhang J, et al. Contribution of IVIM to conventional dynamic contrast-enhanced and diffusion-weighted MRI in differentiating benign from malignant breast masses. Breast Care (Basel) 2016;11(4):254–258. doi: 10.1159/000447765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woodhams R, Kakita S, Hata H, et al. Diffusion-weighted imaging of mucinous carcinoma of the breast: evaluation of apparent diffusion coefficient and signal intensity in correlation with histologic findings. AJR Am J Roentgenol. 2009;193(1):260–266. doi: 10.2214/AJR.08.1670. [DOI] [PubMed] [Google Scholar]
- 134.Xie T, Zhao Q, Fu C, et al. Differentiation of triple-negative breast cancer from other subtypes through whole-tumor histogram analysis on multiparametric MR imaging. Eur Radiol. 2019;29(5):2535–2544. doi: 10.1007/s00330-018-5804-5. [DOI] [PubMed] [Google Scholar]
- 135.Yabuuchi H, Soeda H, Matsuo Y, et al. Phyllodes tumor of the breast: correlation between MR findings and histologic grade. Radiology. 2006;241(3):702–709. doi: 10.1148/radiol.2413051470. [DOI] [PubMed] [Google Scholar]
- 136.Yoo H, Shin HJ, Baek S, et al. Diagnostic performance of apparent diffusion coefficient and quantitative kinetic parameters for predicting additional malignancy in patients with newly diagnosed breast cancer. Magn Reson Imaging. 2014;32(7):867–874. doi: 10.1016/j.mri.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 137.Youk JH, Son EJ, Chung J, et al. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes. Eur Radiol. 2012;22(8):1724–1734. doi: 10.1007/s00330-012-2425-2. [DOI] [PubMed] [Google Scholar]
- 138.Zhang M, Horvat JV, Bernard-Davila B, et al. Multiparametric MRI model with dynamic contrast-enhanced and diffusion-weighted imaging enables breast cancer diagnosis with high accuracy. J Magn Reson Imaging. 2019;49(3):864–874. doi: 10.1002/jmri.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao J, Guan H, Li M, et al. Significance of the ADC ratio in the differential diagnosis of breast lesions. Acta Radiol. 2016;57(4):422–429. doi: 10.1177/0284185115590286. [DOI] [PubMed] [Google Scholar]
- 140.Zhao M, Fu K, Zhang L, et al. Intravoxel incoherent motion magnetic resonance imaging for breast cancer: a comparison with benign lesions and evaluation of heterogeneity in different tumor regions with prognostic factors and molecular classification. Oncol Lett. 2018;16(4):5100–5112. doi: 10.3892/ol.2018.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou J, Chen E, Xu H, et al. Feasibility and diagnostic performance of Voxelwise computed diffusion-weighted imaging in breast Cancer. J Magn Reson Imaging. 2018. 10.1002/jmri.26533. [DOI] [PMC free article] [PubMed]
- 142.Qu RF, Guo DR, Chang ZX, et al. Differential diagnosis of benign and malignant breast Tumors using apparent diffusion coefficient value measured through diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr. 2015;39(4):513–522. doi: 10.1097/RCT.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 143.Liu L, Long M, Wang J, et al. Quantitative analysis of diffusion-weighted imaging for diagnosis of puerperal breast abscess after polyacrylamide hydrogel augmentation mammoplasty: compared with other conventional modalities. Aesthet Plast Surg. 2015;39(1):84–90. doi: 10.1007/s00266-014-0442-z. [DOI] [PubMed] [Google Scholar]
- 144.Guo Y, Kong QC, Zhu YQ, et al. Whole-lesion histogram analysis of the apparent diffusion coefficient: evaluation of the correlation with subtypes of mucinous breast carcinoma. J Magn Reson Imaging. 2018;47(2):391–400. doi: 10.1002/jmri.25794. [DOI] [PubMed] [Google Scholar]
- 145.Surov A, Clauser P, Chang YW, et al. Can diffusion-weighted imaging predict tumor grade and expression of Ki-67 in breast cancer? A multicenter analysis. Breast Cancer Res. 2018;20(1):58. doi: 10.1186/s13058-018-0991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baxter GC, Graves MJ, Gilbert FJ, Patterson AJ. A Meta-analysis of the diagnostic performance of diffusion MRI for breast lesion characterization. Radiology. 2019;291(3):632–641. doi: 10.1148/radiol.2019182510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.