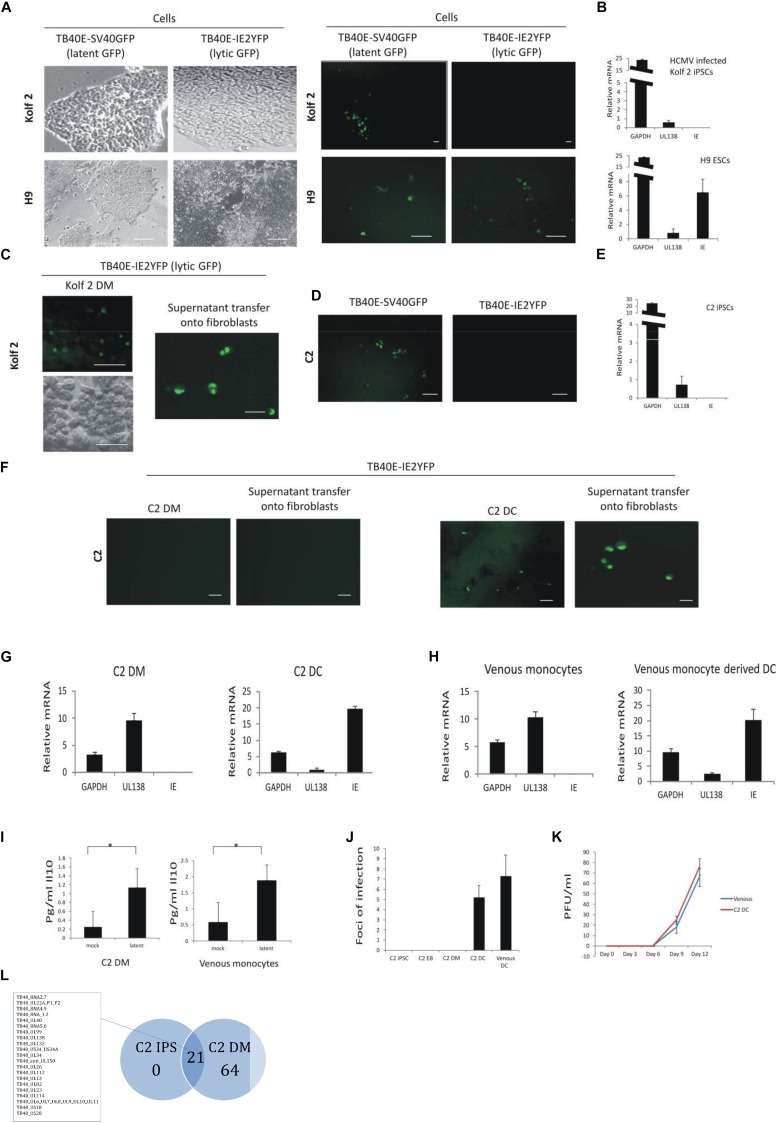
FIGURE 1.
C2 and Kolf2 cells can support a latent infection but only C2 cells maintain latent carriage down the myeloid lineage and can support viral reactivation. Kolf2 and H9 were infected with either TB40E-SV40GFP (MOI 5) (which results in GFP expression in both lytically and latently infected cells) or TB40E-IE2YFP (MOI 5) (which results in GFP expression only in lytically infected cells) and analyzed by fluorescence microscopy at 4 days post infection (brightfield and fluorescence images are shown) (A) or RT-qPCR of the SV40GFP (MOI 5) infected cells (B). Additionally, after differentiation to EBs, monocytes derived from Kolf2 cells were infected with TB40E-IE2YFP virus (MOI 5) (C, left panel) and supernatants were transferred after 8 days to fibroblasts and analyzed by IF 6 days later (C, right panel). C2 iPSCs were also infected with TB40E-SV40GFP (MOI 5) or TB40E-IE2-YFP (MOI 5) and analyzed by fluorescent microscopy (D) or RNA qPCR (E). After differentiation to EBs, the derivative C2 iPSC-derived monocytes (C2 DMs) were infected with TB40E-IE2YFP (MOI 5) virus (F, left hand panels) and these cells were subsequently differentiated to C2 DCs for 8 days (F, right hand panels) and examined for GFP fluorescence directly or co-cultured on indicator fibroblasts as indicated. Alternatively, latently infected C2 DMs were analyzed directly by RT-qPCR (G, left panel) or after their differentiation to C2 DCs (G, right panel). Latently infected venous monocytes were similarly analyzed directly by RT-qPCR (H, left panel) or after their further differentiation to DCs (H, right panel). Supernatants from C2 DMs or venous monocytes that had been latently infected with TB40E-SV40GFP (MOI 5) were also analyzed for cellular IL-10 induction by ELISA (I). C2 cells were infected with TB40E-IE2YFP (MOI 5) and then supernatants transferred to fibroblasts at the different stages of myeloid differentiation as indicated. Venous monocytes latently infected with TB40E-IE2YFP (MOI 5) were also differentiated for 8 days to DCs (venous DCs) before transferring supernatants to fibroblasts and foci of infection (indicating reactivation) were enumerated 6 days post-transfer (J). Finally, C2 DMs and venous monocytes (venous) were infected with HCMV (MOI 5) for 4 days before differentiating into DCs and transferring supernatants onto fibroblasts for up to 12 days. Supernatants from these were then used to inoculate fresh fibroblasts to assay virus release (K). Standard error bars are shown and significance determined using Student’s t-test where ∗p-value < 0.05. In photomicrographs, the bar represents 50 μm. Finally, undifferentiated C2 iPSC cells as well as C2 DMs were latently infected with HCMV for 5 days before sorting and analyzing by single cell RNAseq. The average number of reads from 130 latent C2 and 32 latent C2 DMs were averaged and each viral product with a read over 0.05 was enumerated and presented in as a Venn diagram and genes common to latency in both cell types are listed (L). The full dataset is shown in Supplementary Figure 3E. The list of genes common to latency in both cells types are listed.