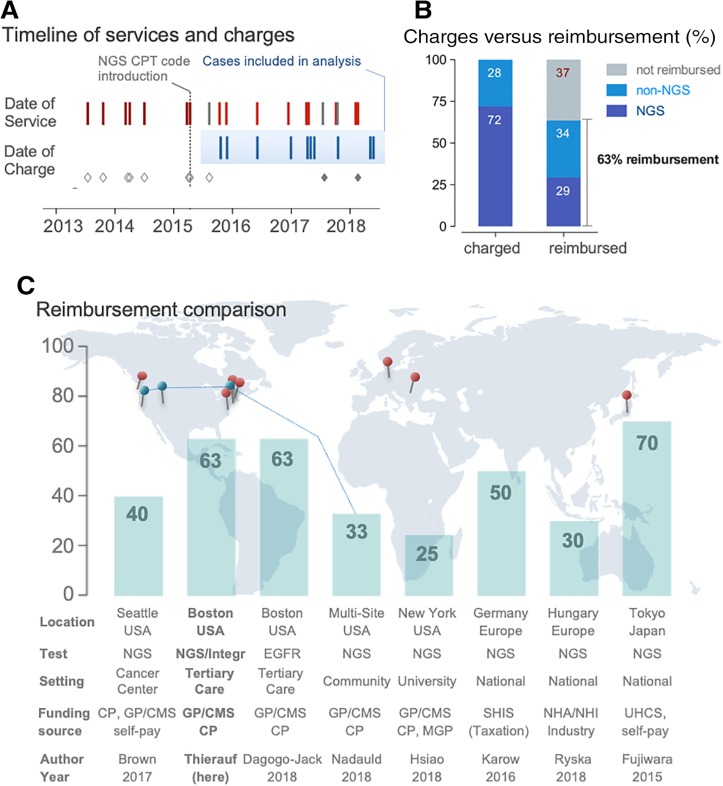
Figure 4.
Financial sustainability of adenoid cystic carcinoma testing in clinical practice. (A): Timeline shows dates of service and dates of charges. In 2015, CPT codes for NGS‐based panel testing were available and introduced (vertical line). We excluded cases with miscellaneous CPT codes (applied before availability of NGS codes indicated by open gray diamonds) and those payed for from alternative sources (indicated by filled gray diamonds). The cases included in the reimbursement analysis span 2.5 years. (B): Bar graphs comparing charged (left) and reimbursed amounts (right) separated by NGS‐ and non‐NGS related fractions. Note: The lower rate in the fraction of reimbursement of NGS codes when compared with non‐NGS codes is likely related to a combination of (a) lower rates of payments, (b) denials, and/or (c) a relative larger fraction of payments related to non‐NGS codes. (C): Bar graphs indicating reimbursement in percent; the location of the eight references (see Results) is provided on the world map.
Abbreviations: Author, first author of the publication; CP, commercial plans; CPT, current procedural terminology; EGFR, epidermal growth factor receptor (single gene testing); GP/CMS, government plans/Center for Medicare and Medicaid services; MGP, Managed government plans (i.e., Medicaid health maintenance organizations and managed Medicaid/Medicare plans); NGS, next‐generation sequencing; NHA/NHI, national health authority/national health insurance; SHIS, Germany's statutory health insurance system; UHCS, Japanese universal health care system.