Figure 1.
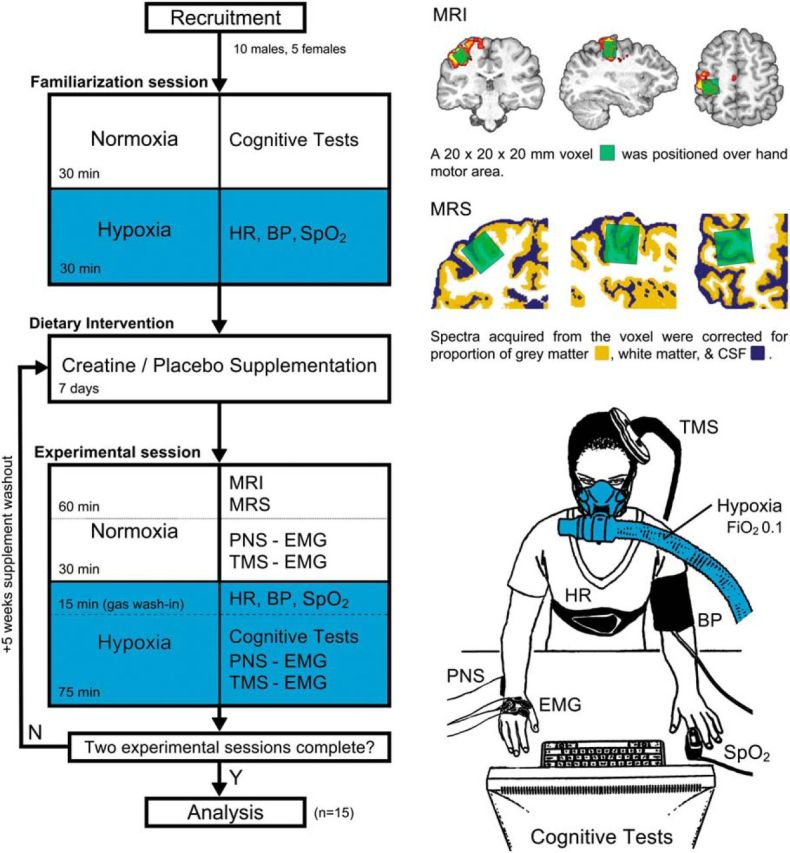
Experimental workflow and procedures. Fifteen healthy participants were recruited to participate in the study. A familiarization session was conducted to collect the baseline neuropsychological assessment (Cognitive Tests) and introduce the participant to the hypoxia intervention (Hypoxia). Participants were then randomized into an initial supplementation regime (CrM or PLA), and supplementation was conducted for 7 d, with a 5 week washout period. Experimental sessions were conducted 24 h after supplementation, involving the collection of neuroimaging, neurophysiological, and neuropsychological data. Structural MRI was used to position an MRS volume of interest (voxel) over the hand knob of the primary motor cortex. The location was verified with functional MRI. The red–yellow contrast shows areas of increased activation during a functional finger-tapping task (Z > 2.3, p < 0.05). Spectroscopy data were acquired and corrected for the proportion of tissue types using neural tissue segmentation and transformed to absolute quantities using a brain-mimicking phantom containing a range of known physiological Cr concentrations. Neurophysiological data, as assessed via TMS and PNS, were recorded using surface EMG at baseline and during the hypoxia intervention to measure central and peripheral excitability levels, respectively. The hypoxia intervention was conducted for 90 min using a gas mixture with a fraction of inspired oxygen (FiO2) of 0.1 and delivered via a one-way valve face-mask system. Cardiovascular measures [arterial oxygen saturation (SpO2), heart rate (HR), and blood pressure (BP)] were monitored throughout to assess autonomic system regulation to the hypoxic intervention.
