Figure 3.
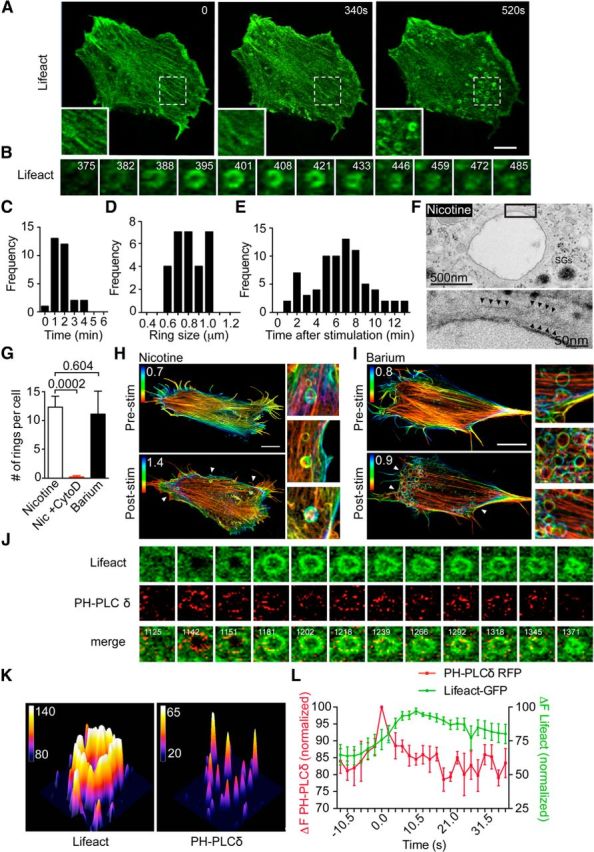
PtdIns(4,5)P2 microdomains precede the appearance of actin rings. A, Bovine adrenal chromaffin cells transfected with Lifeact-GFP were imaged at the level of the footprint cortical actin network by time-lapse confocal microscopy before and during nicotine stimulation (100 μm). Scale bar, 5 μm. The three panels are indicative of the change in the cortical actin network occurring during stimulation, including a partial depolymerization/remodeling followed by the appearance of actin rings in some areas. B, Time series of a single actin ring from the cell shown in A highlighting its formation and constriction (time after stimulation in seconds is indicated in the panels). C–E, Quantitative analysis of actin ring size (C), duration (D), and time of appearance following the onset of stimulation [E; n = 30 rings from six cells (C, D); n = 75 rings from six cells (E)]. F, Bovine chromaffin cells were treated with nicotine (100 μm) for 2 min and were processed for electron microscopy. Nicotine stimulation triggered the appearance of large membrane-bound endosomes, a subset of which were surrounded by a detectable cytoskeleton (arrowheads). G, Bovine chromaffin cells transfected with Lifeact-GFP were imaged following stimulation with either nicotine (100 μm) in the presence or absence of cytochalasin D (10 μm, 30 min preincubation) or with BaCl2 (2 mm), and the number of rings per cell was analyzed (n = 6 and n = 8 cells, respectively). H, I, Bovine chromaffin cells transfected with Lifeact-GFP were imaged using SIM, and z-stack images were acquired before and after stimulation with either nicotine (100 μm; H) or BaCl2 (2 mm; I). The color scale indicates the distance from the plasma membrane to the value indicated in the panels (micrometers in z-stack). Scale bar, 5 μm. J, Chromaffin cells cotransfected with Lifeact-GFP and PH-PLCδ-RFP were time lapse imaged (0.5 Hz) during stimulation with nicotine (100 μm). The panels show a region of interest highlighting the appearance of highly intense PH-PLCδ-positive microdomains preceding an individual actin ring. K, 3D surface plot of Lifeact-GFP ring and PH-PLCδ-RFP-positive microdomains taken from G 1218 s after stimulation. Note that the intensity of the actin ring appears to be continuous, whereas those of the PtdIns(4,5)P2 microdomains are discrete. L, Analysis of the time course of Lifeact-GFP and PH-PLCδ-RFP fluorescence intensity at the level of identified actin rings. To measure the latency of actin ring formation, the peak intensity of PH-PLCδ-RFP was used as a time reference, and its intensity as normalized. Note that the PtdIns(4,5)P2 signal precedes actin ring formation (n = 6 rings from three cells). Error bars are mean ± SEM.
