Figure 6.
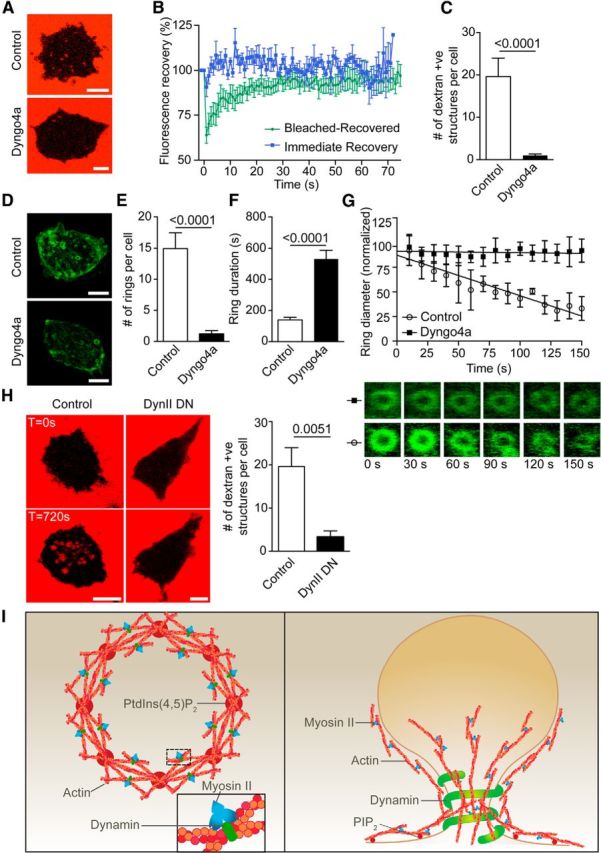
Dynamin inhibition prevents actin ring formation and dextran-positive endosomal fission. A, Bovine adrenal chromaffin cells were incubated with 70 kDa dextran-TMRh in the presence or absence of Dyngo4a (30 μm) and were imaged by time-lapse confocal microscopy during nicotine stimulation (100 μm). Scale bar, 5 μm. B, Dextran-positive structures formed following stimulation in the presence of Dyngo4a were photobleached using 100% laser stimulation (20 iterations) and were time lapse imaged at 0.5 Hz. All dextran-positive structures fully recovered their fluorescence, indicating persisting continuity with the extracellular dextran (n = 7 Bleached-Recovered dextran-positive structures from four cells; n = 5 Immediate Recovery dextran-positive structures from three cells). C, Analysis of the number of dextran-positive structures appearing following stimulation (n = 6 control cells and n = 29 Dyngo4a-treated cells). D, Chromaffin cells transfected with Lifeact-GFP were stimulated with nicotine (100 μm) in the presence or absence of Dyngo4a (30 μm) and were imaged by time-lapse confocal microscopy. Scale bar, 5 μm. E–G, Analysis of the number of rings formed per cell (E; n = 12 control cells; n = 35 Dyngo4a-treated cells), their duration (F; n = 52 rings from 8 control cells; n = 20 rings from 4 Dyngo4a-treated cells), and their rate of constriction (G; n = 4 rings from 3 control cells; n = 4 rings from 2 Dyngo4a-treated cells). Note that Dyngo4a significantly blocks the number of ring forming and prevent their constriction. H, Chromaffin cells transfected with dynamin II (K44A) dominant-negative mutant were incubated with 70 kDa dextran-TMRh and incubated with or without nicotine (100 μm) while being imaged by time-lapse confocal microscopy at the level of the footprint (surface area in contact with the coverslip). Numerous internal dextran-positive structures appeared after stimulation in control but are significantly reduced in cells expressing Dynamin mutant. Scale bars, 5 μm. I, Left, Model highlighting the initiating role of PtdIns(4,5)P2 in generating actin filaments from regularly spaced hotspots in the plasma membrane delineating the future actin ring. In this model, myosin II and dynamin stabilize the actin ring by cross-linking the intersecting fibers (inset). Right, Model highlighting the role of actin rings in generating bulk endosomes. Myosin II and dynamin play critical roles in generating and stabilizing the actin ring in addition to the role of dynamin in pinching off the nascent bulk endosomes from the plasma membrane.
