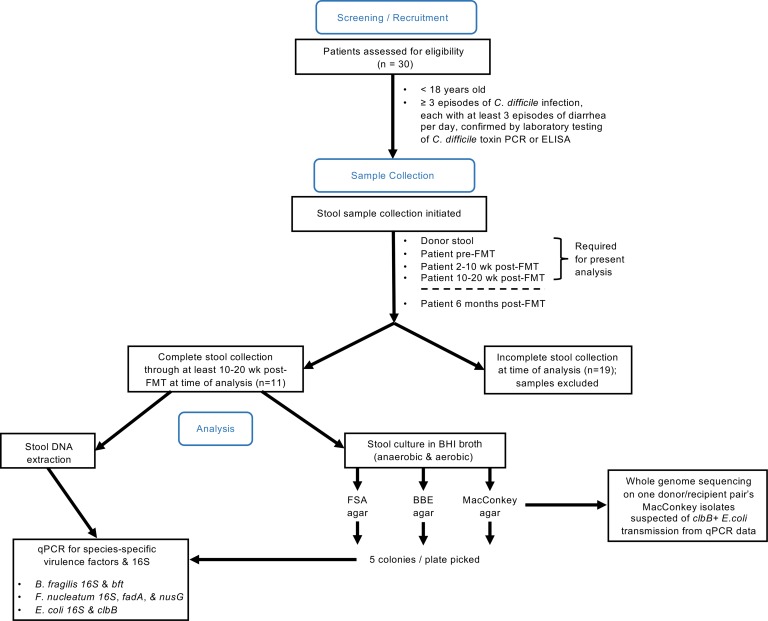
Figure 1. Flow diagram of study design.
Screening/recruitment: pediatric patients (<18 years old) with at least 3 episodes of C. difficile infection were recruited (n = 30 donor/patient pairs). Each episode consisted of at least 3 episodes of diarrhea per day and was confirmed by laboratory test of C. difficile toxin by PCR or ELISA. Sample collection: stool samples were collected from donors and patients at the designated time points. Analysis: at the time of analysis, only patient/donor pairs with a complete set of stool samples collected through at least the 10–20 weeks post-FMT time point were included for further study (n = 11 donor/patient pairs).