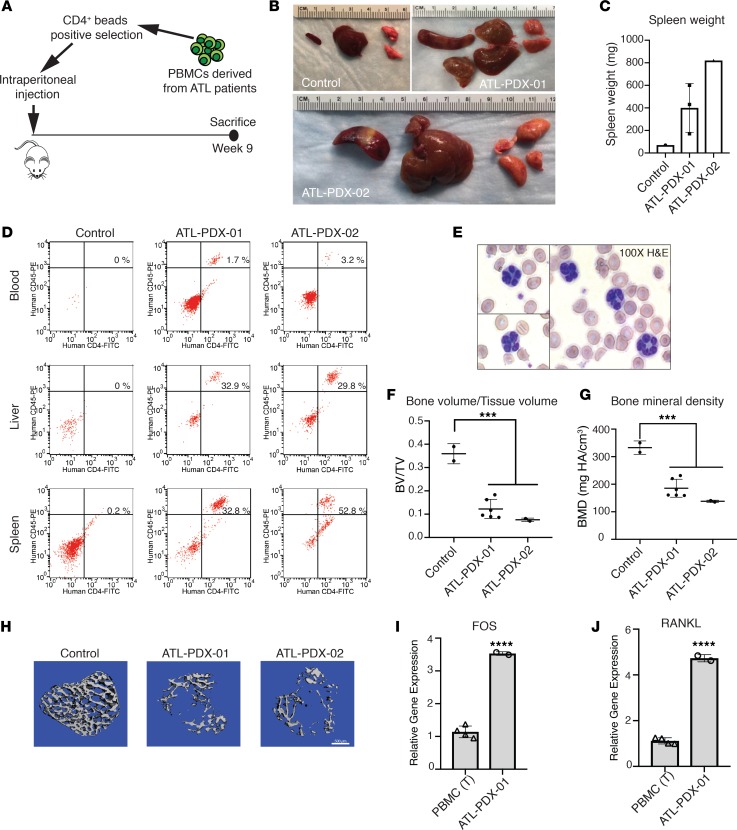
Figure 6. ATL patient–derived xenografts exhibit marked osteolytic bone loss and increased RANKL expression.
(A) Experimental design: CD4+ primary tumor cells derived from the peripheral blood of 2 acute ATL patients (ATL-PDX-01 and ATL-PDX-02) were purified and injected into NBSGW mice by i.p. injection; 1 uninjected NBSGW mouse was used as control; mice were sacrificed at week 9 after inoculation (n = 1–3 per group). (B) ATL-PDX–bearing mice presented with enlargement of spleen, liver, and lung. (C) Increased spleen mass is shown. (D) Flow cytometry for human CD45+CD4+ ATL cells in the blood and spleen. (E) The presence of “flower cells” in peripheral blood smears in mice inoculated with ATL-PDX-02 cells. (F and G) μCT analysis for calculation of tibial trabecular bone to tissue volume ratio (BV/TV) and bone mineral density (BMD). (H) Representative images of 3-D reconstruction of tibial trabecular bone from control and ATL-PDX mice. Scale bar: 500 μm. (I) In vitro qPCR detection of FOS and (J) RANKL RNA in primary ATL cells (ATL-PDX-01), compared with T cells from PBMCs. Data is representative of 2 biological replicates. Error bars in this figure represent ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (2-tailed distribution, homoscedastic Student’s t test for 2 groups or 1-way ANOVA for multiple comparison).