Abstract
One hundred human-derived coagulase negative staphylococci (CoNS) were screened for antimicrobial activity using agar-based deferred antagonism assays with a range of indicator bacteria. Based on the findings of the screen and subsequent well assays with cell free supernatants and whole cell extracts, one strain, designated CIT060, was selected for further investigation. It was identified as Staphylococcus capitis and herein we describe the purification and characterisation of the novel bacteriocin that the strain produces. This bacteriocin which we have named capidermicin was extracted from the cell-free supernatant of S. capitis CIT060 and purified to homogeneity using reversed-phase high performance liquid chromatography (RP-HPLC). Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometric (MS) analysis revealed that the capidermicin peptide has a mass of 5,464 Da. Minimal inhibitory concentration (MIC) experiments showed that capidermicin was active in the micro-molar range against all the Gram-positive bacteria that were tested. Antimicrobial activity was retained over a range of pHs (2–11) and temperatures (10–121°C x 15 mins). The draft genome sequence of S. capitis CIT060 was determined and the genes predicted to be involved in the biosynthesis of capidermicin were identified. These genes included the predicted capidermicin precursor gene, and genes that are predicted to encode a membrane transporter, an immunity protein and a transcriptional regulator. Homology searches suggest that capidermicin is a novel member of the family of class II leaderless bacteriocins.
Introduction
The rise and spread of multidrug-resistant bacterial pathogens, coupled with a diminishing repertoire of effective antibiotics has necessitated the search for new alternative antimicrobial agents. Over the past decade, ribosomally synthesized natural peptides produced by a diverse group of bacterial species have received attention [1]. Compared to non-ribosomally synthesized antimicrobials, the ribosomally produced peptides are attractive for pharmaceutical applications as they could potentially be bioengineered to improve characteristics such as potency, stability, solubility etc. [2]. One group of compounds, namely the bacteriocins, have attracted great interest due to their high potency (often active in the nanomolar range) and heat stability [3].
It has been suggested that most bacteria produce at least one bacteriocin [4]. While the exact ecological function of bacteriocins is unknown, they may play a role in competition by directly killing competing bacteria, function as colonizing peptides or function as signalling molecules to communicate with other bacteria or the host [5]. Bacteriocin-producing bacteria have been isolated from a wide variety of sources including food [6], soil [7] and the intestines of fish and animals [8, 9]. In addition, several members of the human microbiota have been shown to produce bacteriocins. For example we have previously employed both functional and in silico approaches in our laboratory to identify novel bacteriocins from the human gut microbiome [10–15]. Skin-derived bacteria have also been shown to produce, or shown potential to produce, bacteriocins [16–21].
For the current study, we have focused on examining the antimicrobial ability of coagulase negative staphylococci (CoNS) that were isolated from human skin or human blood with the aim of identifying novel bacteriocin-producers. CoNS are considered part of the normal commensal bacteria of the skin and are thought to act as host guardians by targeting pathogens [22]. We postulate that bacteriocin production plays an important role in this function and in support of this hypothesis Nakatsuji and colleagues [23] have shown that antimicrobial-producing CoNS are deficient in subjects with atopic dermatitis and reintroduction of these strains decreased colonization with Staphylococcus aureus. While numerous lantibiotics have been identified from CoNS, including epidermin from Staphylococcus epidermidis, gallidermin from Staphylococcus gallinarium and hominicin from Staphylococcus hominis [24], analysis of the entire genome sequences of publicly available CoNS genomes suggests that there are potentially many novel CoNS-derived bacteriocins that have not yet been functionally characterized (M. Begley; unpublished data).
The aim of this study was to screen one hundred human-derived CoNS for antimicrobial activity using agar-based deferred antagonism assays with a range of indicator bacteria. One CoNS strain (CIT060) was selected for further investigation and it was identified as Staphylococcus capitis and herein we describe the purification and characterisation of the novel bacteriocin that the strain produces.
Materials and methods
Bacterial strains and growth conditions
The bacterial indicator strains used in this study are listed in Table 1. Strains were grown in either Brain Heart Infusion (BHI) broth at 37°C or in M17 broth supplemented with 0.5% glucose (GM17) at 30°C as indicated in the Table 1. All media was purchased from Oxoid and prepared according to the manufacturer’s recommendations.
Table 1. Bacterial strains used in this study.
| Bacterial strains | Culture medium and temperature | Source |
|---|---|---|
| 100 CoNS isolates | BHI at 37°C | CIT Culture Collection |
|
Staphylococcus epidermidis TU3298 Positive control (epidermin producer), used during antimicrobial screening |
BHI at 37°C | Teagasc, Culture Collection |
| Staphylococcus capitis CIT060 | BHI at 37°C | CIT Culture Collection |
| Indicator Strains | ||
| Bacillus cereus DPC 6089 | BHI at 37°C | UCC culture Collection |
| Enterococcus faecailis MR103 | BHI at 37°C | UCC culture Collection |
| Geobacillus kaustophilus DSM 7263 | BHI at 55°C | UCC culture Collection |
| Geobacillus stearothermophilus ATCC 12930 | BHI at 55°C | UCC culture Collection |
| Lactococcus cremoris IP5 | GM17 at 30°C | UCC culture Collection |
| Lactococcus lactis HP | GM17 at 30°C | UCC culture Collection |
| Lactococcus lactis MG1363 | GM17 at 30°C | UCC culture Collection |
| Lactococcus lactis NZ9800 | GM17 at 30°C | UCC culture Collection |
| Micrococcus luteus DSM1790 | BHI at 37°C | UCC culture Collection |
| Staphylococcus aureus 5971 | BHI at 37°C | UCC culture Collection |
| Staphylococcus aureus DPC 5243 | BHI at 37°C | UCC culture Collection |
| Staphylococcus aureus DPC5297 | BHI at 37°C | UCC culture Collection |
| Staphylococcus aureus NCDO 1499 | BHI at 37°C | UCC culture Collection |
| Staphylococcus aureus Newman | BHI at 37°C | UCC culture Collection |
| Staphylococcus aureus RF122 | BHI at 37°C | UCC culture Collection |
| Staphylococcus epidermidis (UCC strain) | BHI at 37°C | UCC culture Collection |
| Staphylococcus gallinarium 4616 | BHI at 37°C | UCC culture Collection |
| Staphylococcus intermedius DSM 20373 | BHI at 37°C | UCC culture Collection |
| Staphylococcus lugdunesis | BHI at 37°C | UCC culture Collection |
| Staphylococcus pseudintermedius DSM 21284 | BHI at 37°C | UCC culture Collection |
| Streptococcus agalactiae ATCC 13813 | BHI at 37°C | UCC culture Collection |
| Streptococcus dysgalactiae ATCC43078 | BHI at 37°C | UCC culture Collection |
| Streptococcus pneumoniae (UCC strain) | BHI at 37°C | UCC culture Collection |
| Streptococcus pyogenes NCDO 2381 | BHI at 37°C | UCC culture Collection |
BHI = Brain heart infusion; GM17 = M17 broth + 0.5% glucose; CIT = Cork Institute of Technology; UCC = University College Cork.
Assembly of a bank of coagulase negative Staphylococci (CoNS)
The 100 CoNS strains were originally isolated from human skin swabs (obtained by swabbing the retro auricular crease i.e. behind the ear, the alar crease i.e. the side of the nose or the wrist), or from human blood samples at Cork University Hospital (CUH). Aliquots from archived stocks that are stored at -80°C at Cork Institute of Technology (CIT) were plated onto Mannitol Salt Agar (MSA) and incubated at 37°C for 16–18 hours. Single colonies were selected from the MSA plates and re-streaked onto BHI agar for purity determination. Strains were identified using Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight (MALDI-TOF) at CUH. Briefly, single fresh colonies (from BHI agar plates incubated at 37°C for 16–18 hours) were directly applied to the MALDI-TOF stainless steel target plate. After application, each bacterial colony was covered with 0.8 μL of matrix solution (10 mg/mL α-cyano-4-hydroxycinnamic acid [HCAA] in 50% acetonitrile-2.5% trifluoroacetic acid) (Bruker Daltonik, GmbH, Germany). The data collected was classified in accordance to Bruker Taxonomy database of CUH.
The 100 strains were re-stocked in two separate master 96 well plates (50 in each plate). Overnight cultures of the 100 strains were prepared by selecting a single pure colony from BHI agar and adding it to 10ml of BHI broth and incubating at 37°C for 16–20 hours. After incubation, 100μl of each fresh overnight culture was added to a specific well in the 96 well plate, and 100μl of sterile 80% glycerol was added to each well. The master stock 96 well plates were stored at -80°C. Prior to use, the plates were thawed at room temperature.
Screening of a bank of CoNS for antimicrobial activity
Agar-based deferred antagonism assays were carried out with a selection of indicator bacteria (listed in Table 1). The 100 CoNS strains were replicated onto BHI agar from the 96 well stock plates using a 96-pin replicator (Boekel). Plates were incubated at 37°C for 16–18 hours after which the surface of the agar plate was subjected to UV treatment for 30 minutes (High performance UV transmitter, Upland, Ca, USA). 30μL of fresh overnight cultures of indicator strains were added to 20ml of relevant sloppy/soft agar (BHI/GM17 broth supplemented with 0.75% w/v agar) and poured over the replicated plates. Plates were incubated at 37°C for 12–16 hours after which they were examined for zones of inhibition.
Investigation of the antimicrobial activity of cell-free supernatants and crude whole cell extracts
Short-listed CoNS were grown overnight in BHI broth at 37°C under vigorous continued shaking (130 rpm), and a 1% inoculum of the cultures were added to 50 ml clarified BHI broth (prepared by passing BHI broth through XAD-16N beads (Sigma-Aldrich) prior to autoclaving) and incubated with vigorous continued shaking at 37°C. Following incubation, 50 ml of bacterial cells were centrifuged at 7,000 rpm for 20 minutes, supernatant removed and retained, i.e., cell-free supernatant (CFS). The cell pellets were resuspended in 7 ml 70% isopropanol (IPA) 0.1% trifluoroacetic acid (TFA) and stirred vigorously for 3 hours. Cell debris was removed through centrifugation and the supernatants were retained and referred to as whole cell extracts (WCE). CFS and WCE were examined for antimicrobial activity in an agar well diffusion assay using L. lactis HP as the indicator strain. 50ml of molten agar was seeded with 100 μL of L. lactis HP that was grown overnight at 30°C, and once solidified, 4.6mm holes were bored with a sterile glass pipette. 50 μL of CFS and WCE were added to separate wells. Plates were incubated at 30°C for 16–18 hours after which they were examined for zones of inhibition.
Purification of capidermicin from S. capitis CIT060
Capidermicin was purified from S. capitis CIT060 using a method described by Field et al. [25] with modifications. Three litres of clarified BHI (cBHI) broth was inoculated (1%) with S. capitis CIT060 and incubated for 18–20 hours at 37°C under vigorous continued shaking (130 rpm). The culture was centrifuged at 7,000 rpm for 15 minutes. The cell pellet was removed, and the supernatant was retained and passed through 60g of Amberlite XAD16N beads (Sigma Aldrich). The beads were washed with 30% ethanol, and the peptide was eluted in 500ml 70% isopropanol (IPA) containing 0.1% trifluoroacetic acid (TFA). The IPA was evaporated using a rotary evaporator (Buchi) and the sample pH adjusted to 4 before applying to a 60 ml Strata C-18 E column (Phenomenex) that was previously pre-equilibrated with 60 ml methanol (Fisher Scientific, UK) and 60 ml H2O. 100 ml of 30% ethanol was used to wash the column and the peptide was eluted in 60 ml of 70% IPA, 0.1% TFA. 10 ml aliquots were concentrated to 2 ml through the removal of IPA by rotary evaporation. 2.0 ml aliquots were applied to a Phenomenex (Phenomenex, Cheshire, UK) C12 reverse phase (RP)-HPLC column (Jupiter 4u proteo 90 Å, 250 × 10.0 mm, 4 μm) previously equilibrated with 25% acetonitrile containing 0.1% TFA. The column was subsequently developed in a gradient of 25% acetonitrile containing 0.1% TFA to 50% acetonitrile containing 0.1% TFA from 10 to 45 minutes at a flow rate of 2ml min-1. The relevant active fractions were collected and pooled, subjected to rotary-evaporation to remove the acetonitrile and freeze-dried. The purity of the peptide was analysed by MALDI-TOF Mass Spectrometry [26].
Minimum inhibitory concentration (MIC) assays
MIC determinations were carried out in triplicate in 96 well microtitre plates pre-treated with bovine serum albumin (BSA) as described by [27]. 200μL of 1X phosphate buffered saline (PBS) containing 1% (w/v) bovine serum albumin (PBS/BSA) was added to each well and incubated for 30 minutes at 37°C. The wells were washed with 200μL PBS and allowed to dry. Target strains were grown overnight in the appropriate medium and temperature conditions, sub-cultured into fresh broth and allowed to grow to an OD600 of approximately 0.5, and diluted to a final concentration of 105 CFU/ml in a volume of 200μL broth. Lyophilised capidermicin was resuspended in cation adjusted BHI broth to a desired concentration, and a 2—fold dilution of the peptide was made in the 96 well plate. The target strain was then added and after incubation at 30°C or 37°C for 16 hours the MIC was read as the lowest peptide concentration causing inhibition of visible growth.
Stability assays with capidermicin
The susceptibility of purified capidermicin peptide to temperature, pH and protease enzymes was investigated through well diffusion assays. To determine temperature stability the purified peptide was subjected to 10, 30, 40, 50, 80, 90, 121°C for 15 minutes. Bioactivity was then determined by carrying out an agar well diffusion assay with L. lactis HP as the bacterial indicator. To evaluate the susceptibility of the peptide to varying pH values, the purified peptide solution was adjusted to pH 2–11 using 1M HCl or 1M NaOH, respectively. After a brief vortex, the peptide was incubated at room temperature for 15 minutes, and the bioactivity was again determined with the well diffusion assay with L. lactis HP. The susceptibility of the peptide to proteolytic cleavage was analysed using trypsin, α-chymotrypsin, pepsin and proteinase K (Sigma-Aldrich). Protease enzymes were dissolved in 100 mM Tris-HCl– 10 mM CaCl2, to a final concentration of 100 μg/ml. Preparations of capidermicin were incubated with the various enzymes at 37°C for 4 hours and the bioactivity of capidermicin was reassessed using the well diffusion assay described above.
Sequencing of S. capitis CIT060 genome
DNA extracted from S. capitis CIT060 was quantified using a Qubit high sensitivity assay (Invitrogen), and diluted to 0.2ng/μl. Genomic libraries were then prepared using the Nextera XT Library preparation kit (Illumina) essentially as described in the manufacturer’s protocol with the following exceptions. Firstly, the tagmentation time was extended to 7min. Following addition of indices, products were cleaned using AMPure XP magnetic bead-based purification, as described in the manufacturer’s protocol and then secondly, in place of the bead based normalisation, the products were run on an Agilent Bioanalyser to determine average fragment size (Agilent) and quantified again by Qubit. Cleaned genomic fragments were then pooled equimolarly. The sample pool (4nM) was denatured with 0.2N NaOH, then diluted to 6pM and combined with 10% (v/v) denatured 6pM PhiX, prepared following Illumina guidelines. Samples were sequenced on the MiSeq sequencing platform in the Teagasc sequencing facility, Moorepark, Fermoy, using a 2 x 300 cycle V3 kit, following standard Illumina sequencing protocols.
In silico analyses of the predicted capidermicin gene cluster and encoded proteins
Following sequencing, the reads were assembled using Spades v. 3.5.0 [28]. Opening reading frames (ORFs) were predicted using Prodigal V.1.20 [29] and assigned a putative function based on BLASTp analysis at NCBI (http://www.ncbi.nlm.nih.gov/) and Pfam matches (EMBL-EBI) [30]. Any genomic regions which were identified as potentially containing antimicrobial-encoding genes were visualised using Snapgene Viewer (GSL Biotech; available at www.snapgene.com). These regions were manually annotated, and BLAST searches were performed with ORFs. Phyre2 (http://www.sbg.bio.ic.ac.uk/~phyre2) [31]) was used to generate a putative three-dimensional structure of the capidermicin peptide using two homologous peptides as templates—aureocin A53 (accession number AAN71834.1) and lacticin Q (accession number BAM66973.1). The sequence alignment of peptides of interest was performed using CLUSTAL OMEGA (https://www.ebi.ac.uk/Tools/msa/clustalo/).
Results
Screening a bank of CoNS strains for antibacterial activity
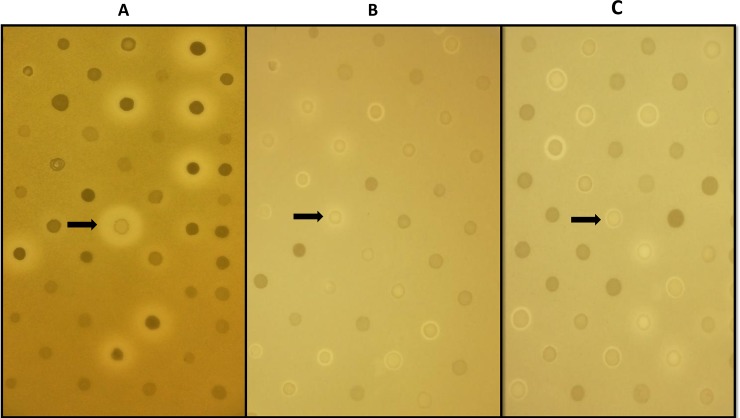
A bank of 100 CoNS strains was assembled. All strains were phenotypically characterised using MSA agar plates and identified by MALDI-TOF analysis. The bank consisted of various staphylococcal species including 83 S. epidermidis, 7 S. capitis, 3 S. haemolyticus, 3 S. hominis, 2 S. warneri, 1 S. saprophyticus and 1 S. simulans. The 100 strains were examined using agar-based deferred antagonism assays for their ability to inhibit a selection of indicator strains (24 in total), including enterococci, lactococci, Micrococcus, streptococci and other Staphylococcus strains. Zones of inhibition were observed for 94 strains; representative images are shown in Fig 1. Six of the S. epidermidis strains did not demonstrate antimicrobial activity under the conditions tested. 15 of the 94 strains displayed a broad spectrum of activity, inhibiting 10 or more indicator strains. One strain, namely S. capitis CIT060, was capable of inhibiting 14 of the 24 bacterial indicators (B. cereus DPC6089, E. faecailis MR103, G. kaustophilius DSM7263, L. lactis subsp cremonis IP5, L. lactis HP, M. luteus DSM1790, S. aureus NCDO1499, DPC5297, Newman, and RF122, S. lugdunensis, S. pseudintermedius DSM21284, S. intermedius DSM 20373 and S. dysgalactiae ATCC43078).
Fig 1. Representative images of the results obtained during the screen of 100 CoNS strains for antimicrobial activity.
CoNS were replicated from master stock 96 well plates onto BHI agar using a 96-pin replicator. Plates were incubated at 37°C overnight after which they were overlaid with sloppy agar containing relevant indicator bacteria. For the plates shown the indicators used were (A) M. luteus DSM1790, (B) S. aureus NCDO1499 and (C) S. pseudintermedius DSM21284. The arrows indicate the position of S. capitis CIT060 on the plates.
Purification of capidermicin from S. capitis CIT060
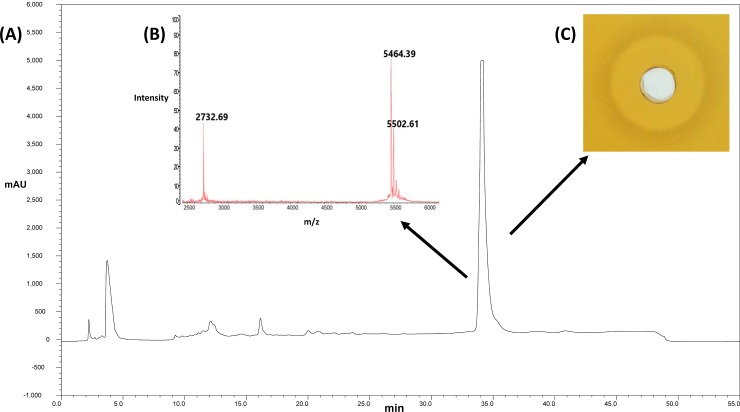
Initial experiments with cell-free supernatant and crude whole cell extracts prepared from S. capitis CIT060 suggested that the antimicrobial produced by the strain is primarily in the supernatant (data not shown). Consequently, efforts focused on purifying an antimicrobial peptide from culture supernatants as described in the Methods section. MALDI-TOF MS analysis revealed that the purified peptide, that we named capidermicin, had a mass of 5,464 Da (Fig 2).
Fig 2.
(A) Reversed–phase high performance liquid chromatography (RP-HPLC) profile for the purification of capidermicin using a Phenomenex C12 reverse-phase column, at a flow rate of 2ml/min. (B) MALDI-TOF Mass Spectrometry of lyophilized capidermicin revealed a mass of 5,464 Da. MALDI TOF MS chromatogram above indicates the presence of capidermicin (5464.39) and the K+ adduct ion (5502.61) and the doubly charged ion (2732.69) (2732 x 2 = 5464). (C) Antimicrobial activity of HPLC bioactive fractions was determined using well diffusion assay using L. lactis HP as the indicator strain. GM17 agar was seeded with L. lactis HP, wells were bored, 50μL of the HPLC fraction was added to the well, and plates were incubated at 30°C for 16 hours.
The antimicrobial activity of purified capidermicin, as determined by agar well diffusion assays with L. lactis HP, remained unaffected following heat treatment at 10, 30, 40, 50, 80, 90 or 121°C for 15 minutes. The peptide was also able to maintain full bioactivity following exposure to pH 2–8. A 70% decrease in zone of inhibition observed at pH 9–11. Exposing capidermicin to α-chymotrypsin and pepsin had no effect on the bioactivity of the peptide. However, treatment with trypsin or proteinase K resulted in a 50% reduction in activity.
The specific activity of capidermicin was assessed using standard MIC broth-based assay and results are presented in Table 2. It was observed that capidermicin was active against the selected target bacteria in the nano- and micromolar range.
Table 2. Minimum inhibitory concentration (MIC) values of purified capidermicin against a range of Gram positive indicators.
Identical MICs values were obtained in three independent determinations.
| Species and Strain | ||
|---|---|---|
| Lactococcus lactis HP | 19 μg/ml | 3.4 μM |
| Staphylococcus aureus NCDO 1499 | 3.1 μg/ml | 0.6 μM |
| Staphylococcus aureus SA113 | 10 μg/ml | 1.8 μM |
| Staphylococcus intermedius DSM20373 | 40 μg/ml | 7.3 μM |
| Staphylococcus pseudintermedius DSM21284 | 10 μg/ml | 1.8 μM |
| Staphylococcus pseudintermedius DK729 | 10 μg/ml | 1.8 μM |
| Micrococcus luteus DSM1790 | 100μg/ml | 18 μM |
In silico analyses of the predicted capidermicin gene cluster and encoded proteins
The draft genome of S. capitis CIT060 was analysed using a variety of in silico tools for the presence of potential antimicrobial peptide encoding genes. Three areas of interest were identified which included a lantibiotic gene cluster, a phenol soluble modulin (PSM) gene cluster and an aureocin-like gene cluster.
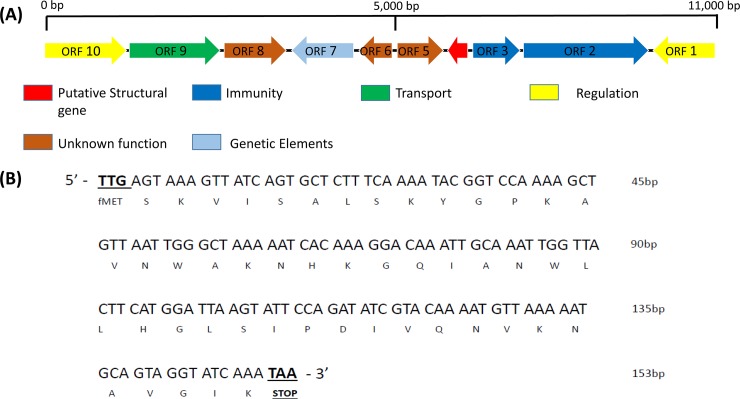
The first area of interest contains a gene that is predicted to encode a 44 amino acid peptide that is homologous to the lantibiotic gallidermin of Staphylococcus gallinarum (accession number U61158.1). The 44 amino acid peptide is predicted to encode a peptide with a mass of 5 kDa. However, a corresponding mass could not be detected from our bioactive HPLC fractions or purified capidermicin preparations. The second area of interest contains four genes that are predicted to encode PSMβ peptides. Phenol-soluble modulins (PSMs) are a recently discovered group of amphipathic peptides that have multiple roles in staphylococcal pathogenesis. They have been shown to exhibit antimicrobial activity [32]. All four predicted peptides contain the conserved domain of staph_haemo superfamilies (pfam05480), their amino acid sequences are identical to the PSMβ peptides previously reported in the literature [16], [21] and they are predicted to have masses of 4.57 kDa, 4.54 kDa, 4.62 kDa and 4.79 kDa. Again, corresponding masses could not be detected from our bioactive HPLC fractions or purified capidermicin preparations. The genetic organization of the final area of interest is shown in Fig 3A and the predicted functions of the putative gene products are shown in Table 3. Orf4 was predicted to encode a peptide that is homologous to a number of previously characterised bacteriocins including lacticin Z produced by Lactococcus lactis QU14 (46% identity; accession number BAF75975), aureocin A53 produced by Corynebacterium jeikeium (41% identity; accession number WP010976360) and an aureocin-like bacteriocin produced by Lactococcus ruminis (57% identity; accession number SEM89646). More distant homologues include BacSp222 (32% identity; accession number A0A0P0C3P7) and Lactolisterin BU (44% identity; accession number SDR48784). A Clustal Omega alignment was carried out to compare the predicted peptide encoded by orf4 and previously characterised bacteriocins, and the relatedness of the peptides is depicted in Fig 4.
Fig 3.
(A) Organisation of the genomic region that is predicted to encode capidermicin. Open reading frames (ORFs)/genes are coloured according to the predicted function. (B) The nucleotide sequence of the 153 bp ORF4 that is predicted to encode capidermicin. The deduced amino acid sequence is shown under the DNA sequence. The start and stop codon, TTG and TAA, respectively, are shown in bold and underlined.
Table 3. In silico analysis of the genes predicted to be involved in the biosynthesis of capidermicin.
| ORF 1 | 60_02323 | 738 | Plasmid replication initiator protein of Listeria monocytogenes | 98% (WP_096929472.1) |
Plasmid replication |
| ORF 2 | 60_02322 | 1407 | YdbT-like protein of S. aureus | 29% (WP_032072953.1) |
Self-immunity |
| ORF 3 | 60_02321 | 405 | YdbS-like protein of S. intermedius | 38% (COG3402) |
Self-immunity |
| ORF 4 | 60_02320 | 153 | Aureocin A53 –like protein | 46% (AF447813) |
Structural gene |
| ORF 5 | 60_02319 | 558 | Membrane protein of Staphylococcus sp.TE8 | 76% WP_082243241.1) |
Unknown |
| ORF 6 | 60_02318 | 243 | Hypothetical protein of S. epidermidis | 95% (WP_049397332.1) |
Unknown |
| ORF 7 | 60_02317 | 624 | Transposase of Staphylococcus | 100% (WP_017176851.1) |
Transposon |
| ORF 8 | 60_02316 | 516 | Hypothetical protein | 99% (WP_020368224.1) |
Unknown |
| ORF 9 | 60_02315 | 996 | Putative sulfate exporter family transporter | 100% (WP_070441690.1) |
Transport of peptide |
| ORF 10 | 60_02314 | 822 | Transcriptional Regulator | 100% (WP_070441693.1) |
Gene Regulation |
Fig 4. Alignment of the capidermicin amino acid sequence with homologous bacteriocins; epidermicin NI01 from S. epidermidis strain 224 (JQ025383), mutacin BHT-B from Streptococcus ratti BHT (DQ145753), lactolisterin BU from L. lactis subsp. lactis bv. diacetylactis BGBU1-4 (SDR48784), lacticin Q from L. lactis QU5 (BAF57910), lacticin Z from L. lactis QU14 (BAF75975), aureocin-like produced by numerous bacteria (SEM89646), and aureocin A53 from S. aureus A53 (WP_010976360).
Highlighted residues indicate conserved amino acid sequences.
The mass of the putative orf4-encoded peptide was predicted to be 5,438 Da by in silico tools (under Genbank accession MN234131). However, as the homologue aureocin A53 contains an N-formylated methionine [33]), and the start codon of orf4 was noted to be TTG (Fig 3B), a revised theoretical mass of 5,466 Da was predicted. This mass is virtually identical to the mass of the capidermicin peptide that we purified from S. capitis CIT060 (Fig 2).
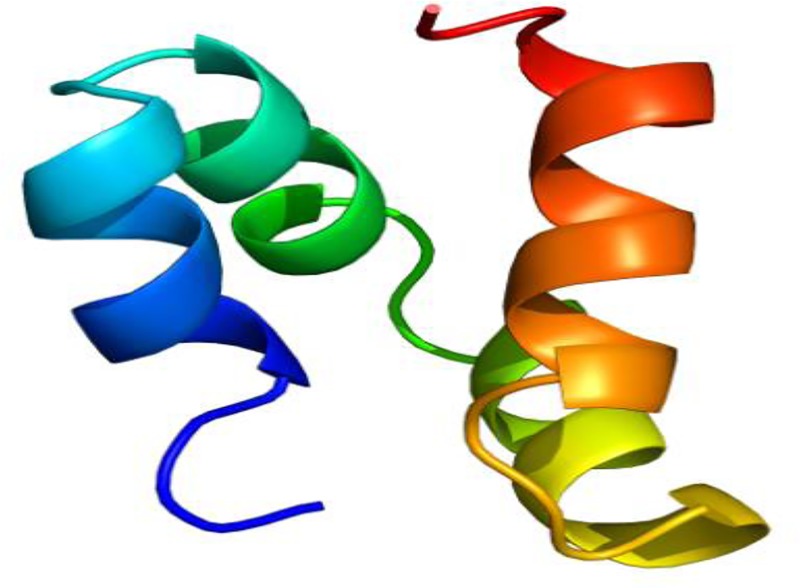
Capidermicin is predicted to be cationic with a putative net charge of 5.34 (theoretical pI = 10.22) and is rich in lysine residues (14%). A 3D structural model is presented in Fig 5. The peptide is predicted to be composed of four α-helices.
Fig 5. Putative three-dimensional structure of capidermicin.
A rainbow colour scheme is used to indicate the N-terminus in blue, and the C-terminus in red. The structure was generated using Phyre2.
Discussion
The aim of the current study was to screen 100 human-derived CoNS for antimicrobial activity. Agar-based deferred antagonism assays revealed that 94 of the strains reproducibly inhibited at least some of the tested indicator bacteria. Six strains did not show antimicrobial activity under the test conditions employed but it is possible that these strains may demonstrate activity under other conditions, such as different growth medium or different indicator bacteria. Janek and colleagues [18] carried out a similar agar-based screen to determine the frequency of antimicrobial production by 89 human nasal Staphylococcus isolates. 77 of the 89 isolates (86.5%) exhibited antimicrobial activity. When taken together, the findings of both studies suggest that antimicrobial production is a common phenotype among CoNS isolates. In contrast, a recent study by O’ Sullivan et al. [19], which describes a similar screen with human skin-derived staphylococci reports that only 101 possible antimicrobial-producers were identified from over 90,000 colonies that were screened for antimicrobial activity. The difference in the frequency of isolation of antimicrobial producers between that study (0.112%), the study by Janek et al. (86.5%) and our study (94%) may be because O’ Sullivan et al. used only one indicator organism in their screen (Lactobacillus delbrueckii subsp. bulgaricus) while a variety of indicators were used in the other two studies. It is possible that antimicrobials produced by CoNS may not inhibit L. delbruecki or activity may be too low to be detected in deferred antagonism assays.
Interestingly, functional screens of human intestine-derived bacteria report isolation of antimicrobial producers at a low frequency. Lakshminarayanan et al. [11] screened over 70,000 faecal bacteria for their ability to inhibit the indicators Lactobacillus bulgaricus LMG6901 and Listeria innocua DPC3572 and only identified 55 antimicrobial producers (a frequency of 0.08%). The in silico-based investigations of Zheng and colleagues [34] revealed that the human gut microbiome had the lowest frequency of putative bacteriocin genes of all the human body sites investigated. It is possible that production of antimicrobials by CoNS will increase their fitness in order to compete and survive on human skin. The limited availability of nutrients and water in this environment compared to the gastrointestinal tract may mean that the skin is a more competitive environment hence explaining the higher frequency of antimicrobial producers in screens with skin-derived bacteria compared to gut-derived bacteria. As humans and their microbes have co-evolved it is likely that production of antimicrobials by CoNS plays a beneficial role for the host, perhaps by contributing to the role of the skin as the body’s first line of defence by protecting against pathogenic bacteria. It is also possible that the antimicrobials may act as signalling molecules and interact with the human immune system [5].
The 94 CoNS that demonstrated antimicrobial activity in the initial screen were shortlisted to 15 based on their inhibition spectra and the activity of cell-free supernatants and whole cell extracts in well assays. S. capitis CIT060 was selected for further investigation as it demonstrated a broad inhibition spectrum and the results of well assays suggested that the antimicrobial could potentially be purified using methods that we currently use for bacteriocins in our lab [25]. We also noted that there are very few reports of functionally characterized S. capitis antimicrobials in the literature. While antimicrobial production by S. capitis strains has been shown by agar-based studies [18, 19] and antimicrobial genes have been identified in S. capitis genomes by in silico based methods [16, 35], to our knowledge the only two functional characterization studies in the literature are those of Sugai et al. [36], who reported the purification of the glycylglycine endopeptidase ALE-1 from S. capitis EPK1 that is similar to the bacteriocin lysostaphin, and Kumar et al. [21] who chemically synthesized and characterized phenol soluble modulins.
A 5,464 Da peptide, that we named capidermicin, was purified from S. capitis CIT060 cell free supernatants. The antimicrobial activity of the peptide was confirmed, and it was shown to retain its activity over a range of pH and temperatures. Analysis of the S. capitis CIT060 genome revealed the presence of 6 potential antimicrobial encoding genes (2 bacteriocins, 4 PSMs) but based on mass we deduced that the antimicrobial peptide that we purified was the product of the aureocin-like gene cluster. A gene encoding a potential 44 amino acid lantibiotic similar to gallidermin was also identified in the genome of S. capitis CIT060. Carson et al. [35] reported the presence of lanthipeptide gene clusters in the genomes of the 12 S. capitis isolates analysed (S. capitis 1319, 3379, 3769, 4275, 6079, 807, 1187, 1642, 2477, 2643, 4830 and 5871). Three of these strains were also shown to contain a sactipeptide gene cluster (S. capitis 1319, 2487 and 3379) [35]). Kumar et al. [21] identified four distinct gene clusters with the ability to encode antimicrobial peptides (epidermicin, gallidermin and phenol-soluble modulins) in S. capitis TE8. While the lantibiotic did not seem to be produced by S. capitis CIT060 under the experimental conditions used, the production of more than one bacteriocin by a bacterium is not uncommon. Lactococci commonly produce more than one bacteriocin and Lactococcus lactis subsp. lactis bv. diacetylactis BGBU1-4 strain produces at least two bacteriocins [37, 38]. Similarly, Staphylococcus aureus 4185, a bovine mastitis isolate, was shown to produce five antimicrobial peptides (named peptides A–E) [39].
In silico analyses suggest that capidermicin is a novel member of the class II leaderless bacteriocin family. It shows most similarity to members of the aureocin 53-like sub-group of the family which includes aureocin A53 produced by S. aureus A53 [33], lacticin Z produced by L. lactis QU14 [40], lacticin Q produced by L. lactis QU5 [41] and epidermicin NI01 produced by S. epidermidis strain 224 [42]. All of the bacteriocins within the group are 34–53 amino acid peptides, are highly cationic and are characterised by their lack of an N-terminal leader sequence during biosynthesis meaning that they do not undergo any post-translational modifications and become active shortly after translation [43]. While the genes encoding the immunity and secretion machinery have been experimentally determined for lacticin Q and Z [44], [45], aureocin A53 [46] and aureocin A70 [47]; [48], compared to other the classes of bacteriocins there is little known about the biosynthesis of leaderless bacteriocins and they have been referred to as the most enigmatic and poorly understood group of bacteriocins [43]. Similar to lacticin Q (48%) and aureocin A53 [49], capidermicin is predicted to be α-helical globular molecule.
MIC assays revealed that capidermicin was active against Gram-positive bacteria at low concentrations (μM/nM). Similar findings have been reported for epidermicin NI01 [42] and lacticin Q [41]. While capidermicin is insensitive to α-chymotrypsin and pepsin, a 70% reduction in activity was observed when it was treated with proteinase K or trypsin. A similar decrease in activity has previously been observed for epidermicin NI01, which displayed a 75% and 50% reduction in activity for proteinase K and trypsin, respectively [42]. Resistance to proteases has been reported for other staphylococcal bacteriocins, including aureocin A53 and BacCH91 [33]; [50]. Capidermicin showed high stability under acidic, alkaline and neutral conditions, which has also been reported for aureocin A53 [33], lacticin Z [40] and lacticin Q [41]). It has previously been noted that the high stability of leaderless bacteriocins together with the simplicity of their biosynthesis may make them more attractive from a commercial view point compared to other bacteriocins [43].
A 3D structural model is presented in Fig 5. The peptide is predicted to be composed of four α-helices, and exhibits a recurring three dimensional structural motif found among many linear leaderless bacteriocins (lacticin Q, aureocin A53) and similar to that found in a larger superfamily of proteins known as saposin-like peptides [51]. All the helices of capidermicin are amphipathic whereby hydrophobic residues are oriented inward that pack to give a hydrophobic core and the hydrophilic residues are exposed on the surface in the same manner as LnqQ (data not shown). Similarly, both peptides have highly cationic surfaces. Capidermicin has 7 lysine residues which are well distributed throughout its primary structure. Indeed, all lysine residues are situated on the surface and are in fact found in the exact same locations to LnqQ (in capidermicin K3, K10, K14, K23, K44 and K50). Notably, although LnqQ and the closely related aureocin A53 are composed of four distinct α-helical structures that are structurally identical to each other, both have varying antimicrobial activity spectrums against Gram-positive bacteria, [49]. Studies have shown that LnqQ permeates target membranes by forming toroidal pores (4.6–6.6 nm in diameter), which facilitate the leakage of cellular contents [52]. However, a more recent study demonstrated that LnqQ was able to induce cell death even without the formation of pores [53]. AucA was also proposed to permeabilize cell membranes causing the leakage of essential molecules, dissipation of membrane potential, and cessation of macromolecular synthesis but without the formation of discrete pores [54]. Given the structural similarity of capidermicin to LnqQ and A53, it is likely that capidermicin can also permeabilize cell membranes resulting in cell death.
In conclusion, we report that our screening experiment revealed a large frequency of antimicrobial production by human CoNS isolates and we describe the subsequent identification and characterization of a novel bacteriocin from S. capitis. Future work in our laboratory will include the chemical synthesis of the capidermicin peptide for additional in vitro experiments to confirm activity and antimicrobial spectrum, similar to studies conducted on other leaderless bacteriocins including garvicin KS and epidermicin NI01 [55, 56]. In the case of epidermicin NI01, chemical synthesis permitted the generation of sufficient peptide for further analyses including in vivo studies [57, 58]. Moreover, accessibility to capidermicin by chemical synthesis would provide a means for peptide engineering investigations to be carried out i.e. molecular engineering to enhance the potency, improve pharmacological properties, increase peptide stability and potentially modify the spectrum of activity. It may also provide more detail regarding the importance of the formylated methionine at the N-terminus for the antimicrobial activity of capidermicin. Future experiments will also include mutational analysis of all of the genes that are predicted to be involved in capidermicin production. A comprehensive gene disruption of the capidermicin biosynthetic cluster will be carried out, similar to that of Iwatani and colleagues [56] whereby each gene of the lacticin Q operon (lnqQBCDEF) was individually deleted and the impact on production and immunity evaluated.
Acknowledgments
We are grateful to Dr. Fiona Crispie (Teagasc, Moorepark) for kindly giving us access to genome sequencing platforms and experimental assistance. We would like to acknowledge the guidance of Dr. Alan Lucid in the annotation of the Staphylococcus capitis CIT060 genome.
Data Availability
The sequence of the S. capitis CIT060 genomic region that was analysed in this study was submitted to Genbank (accession number MN234131).
Funding Statement
This work was supported by a Cork Institute of Technology RISAM PhD scholarship to DL. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30(1):108–60. 10.1039/c2np20085f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez RH, Zendo T, Sonomoto K. Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microbial Cell Factories. 2014;13(Suppl 1):S3–S. 10.1186/1475-2859-13-S1-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter PD, Ross RP, Hill C. Bacteriocins—a viable alternative to antibiotics? Nature Reviews Microbiology. 2013;11(2):95–105. 10.1038/nrmicro2937 [DOI] [PubMed] [Google Scholar]
- 4.Riley MA, Wertz JE. Bacteriocins: Evolution, Ecology, and Application. Annual Review of Microbiology. 2002;56(1):117–37. 10.1146/annurev.micro.56.012302.161024 [DOI] [PubMed] [Google Scholar]
- 5.Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin Production: a Probiotic Trait? Applied and Environmental Microbiology. 2012;78(1):1–6. 10.1128/AEM.05576-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly WJ, Asmundson RV, Huang CM. Isolation and characterization of bacteriocin-producing lactic acid bacteria from ready-to-eat food products. International journal of food microbiology. 1996;33(2–3):209–18. 10.1016/0168-1605(96)01157-9 [DOI] [PubMed] [Google Scholar]
- 7.Yanagida F, Chen YS, Shinohara T. Searching for bacteriocin-producing lactic acid bacteria in soil. J Gen Appl Microbiol. 2006;52(1):21–8. Epub 2006/04/07. . [DOI] [PubMed] [Google Scholar]
- 8.Collins FWJ, O’Connor PM, O'Sullivan O, Rea MC, Hill C, Ross RP. Formicin–a novel broad-spectrum two-component lantibiotic produced by Bacillus paralicheniformis APC 1576. Microbiology. 2016;162(9):1662–71. 10.1099/mic.0.000340 [DOI] [PubMed] [Google Scholar]
- 9.Ringø E, Hoseinifar SH, Ghosh K, Doan HV, Beck BR, Song SK. Lactic Acid Bacteria in Finfish—An Update. Frontiers in Microbiology. 2018;9:1818–. 10.3389/fmicb.2018.01818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begley M, Cotter PD, Hill C, Ross RP. Identification of a Novel Two-Peptide Lantibiotic, Lichenicidin, following Rational Genome Mining for LanM Proteins. Applied and Environmental Microbiology. 2009;75(17):5451–60. 10.1128/AEM.00730-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakshminarayanan B, Guinane CM, O'Connor PM, Coakley M, Hill C, Stanton C, et al. Isolation and characterization of bacteriocin-producing bacteria from the intestinal microbiota of elderly Irish subjects. Journal of Applied Microbiology. 2013;114(3):886–98. 10.1111/jam.12085 [DOI] [PubMed] [Google Scholar]
- 12.O'Connor PM, O'Shea EF, Guinane CM, O'Sullivan O, Cotter PD, Ross RP, et al. Nisin H Is a New Nisin Variant Produced by the Gut-Derived Strain Streptococcus hyointestinalis DPC6484. Applied and Environmental Microbiology. 2015;81(12):3953–60. 10.1128/AEM.00212-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Sullivan O, Begley M, Ross RP, Cotter PD, Hill C. Further Identification of Novel Lantibiotic Operons Using LanM-Based Genome Mining. Probiotics and Antimicrobial Proteins. 2011;3(1):27–40. 10.1007/s12602-011-9062-y [DOI] [PubMed] [Google Scholar]
- 14.Collins FWJ, O’Connor PM, O’Sullivan O, Gómez-Sala B, Rea MC, Hill C, et al. Bacteriocin Gene-Trait matching across the complete Lactobacillus Pan-genome. Scientific Reports. 2017;7(1):3481–. 10.1038/s41598-017-03339-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh CJ, Guinane CM, Hill C, Ross RP, O’Toole PW, Cotter PD. In silico identification of bacteriocin gene clusters in the gastrointestinal tract, based on the Human Microbiome Project’s reference genome database. BMC Microbiology. 2015;15(1):183 10.1186/s12866-015-0515-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron DR, Jiang J-H, Hassan KA, Elbourne LDH, Tuck KL, Paulsen IT, et al. Insights on virulence from the complete genome of Staphylococcus capitis. Frontiers in microbiology. 2015;6:980–. 10.3389/fmicb.2015.00980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 2016;535(7613):511–6. 10.1038/nature18634 [DOI] [PubMed] [Google Scholar]
- 18.Janek D, Zipperer A, Kulik A, Krismer B, Peschel A, Bertram R. High Frequency and Diversity of Antimicrobial Activities Produced by Nasal Staphylococcus Strains against Bacterial Competitors. PLOS Pathogens. 2016;12(8):e1005812–e. 10.1371/journal.ppat.1005812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Sullivan JN, Rea MC, O'Connor PM, Hill C, Ross RP. Human skin microbiota is a rich source of bacteriocin-producing staphylococci that kill human pathogens. FEMS Microbiology Ecology. 2019;95(2). 10.1093/femsec/fiy241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? British Journal of Dermatology. 2008;158(3):442–55. 10.1111/j.1365-2133.2008.08437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R, Jangir PK, Das J, Taneja B, Sharma R. Genome Analysis of Staphylococcus capitis TE8 Reveals Repertoire of Antimicrobial Peptides and Adaptation Strategies for Growth on Human Skin. Scientific reports. 2017;7(1):10447–. 10.1038/s41598-017-11020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen GJ, Bruggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes. 2014;5(2):201–15. Epub 2013/12/11. 10.3920/BM2012.0062 . [DOI] [PubMed] [Google Scholar]
- 23.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Science translational medicine. 2017;9(378):eaah4680–eaah. 10.1126/scitranslmed.aah4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Götz F, Perconti S, Popella P, Werner R, Schlag M. Epidermin and gallidermin: Staphylococcal lantibiotics. International Journal of Medical Microbiology. 2014;304(1):63–71. 10.1016/j.ijmm.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 25.Field D, Gaudin N, Lyons F, O'Connor PM, Cotter PD, Hill C, et al. A Bioengineered Nisin Derivative to Control Biofilms of Staphylococcus pseudintermedius. PLOS ONE. 2015;10(3):e0119684–e. 10.1371/journal.pone.0119684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotter PD, Draper LA, Lawton EM, McAuliffe O, Hill C, Ross RP. Overproduction of wild-type and bioengineered derivatives of the lantibiotic lacticin 3147. Applied and environmental microbiology. 2006;72(6):4492–6. 10.1128/AEM.02543-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field D, Begley M, O’Connor PM, Daly KM, Hugenholtz F, Cotter PD, et al. Bioengineered Nisin A Derivatives with Enhanced Activity against Both Gram Positive and Gram Negative Pathogens. PLoS ONE. 2012;7(10):e46884–e. 10.1371/journal.pone.0046884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. Journal of Computational Biology. 2012;19(5):455–77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11(1):119–. 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Research. 2016;44(D1):D279–D85. 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols. 2015;10(6):845–58. 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung GY, Joo HS, Chatterjee SS, Otto M. Phenol-soluble modulins—critical determinants of staphylococcal virulence. FEMS microbiology reviews. 2014;38(4):698–719. Epub 2014/01/01. 10.1111/1574-6976.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netz DJA, Pohl R, Beck-Sickinger AG, Selmer T, Pierik AJ, Bastos MdCdF, et al. Biochemical Characterisation and Genetic Analysis of Aureocin A53, a New, Atypical Bacteriocin from Staphylococcus aureus. Journal of Molecular Biology. 2002;319(3):745–56. 10.1016/S0022-2836(02)00368-6 [DOI] [PubMed] [Google Scholar]
- 34.Zheng J, Gänzle MG, Lin XB, Ruan L, Sun M. Diversity and dynamics of bacteriocins from human microbiome. Environmental Microbiology. 2015;17(6):2133–43. 10.1111/1462-2920.12662 [DOI] [PubMed] [Google Scholar]
- 35.Carson DA, Barkema HW, Naushad S, De Buck J. Bacteriocins of non-aureus staphylococci isolated from bovine milk. Applied and environmental microbiology. 2017:AEM.01015-17. 10.1128/AEM.01015-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugai M, Fujiwara T, Akiyama T, Ohara M, Komatsuzawa H, Inoue S, et al. Purification and molecular characterization of glycylglycine endopeptidase produced by Staphylococcus capitis EPK1. Journal of bacteriology. 1997;179(4):1193–202. 10.1128/jb.179.4.1193-1202.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miljkovic M, Uzelac G, Mirkovic N, Devescovi G, Diep DB, Venturi V, et al. LsbB Bacteriocin Interacts with the Third Transmembrane Domain of the YvjB Receptor. Applied and Environmental Microbiology. 2016;82(17):5364–74. 10.1128/AEM.01293-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojic M, Strahinic I, Fira D, Jovcic B, Topisirovic L. Plasmid content and bacteriocin production by five strains of Lactococcus lactis isolated from semi-hard homemade cheese. Can J Microbiol. 2006;52(11):1110–20. Epub 2007/01/12. 10.1139/w06-072 . [DOI] [PubMed] [Google Scholar]
- 39.Ceotto H, Brede D, Salehian Z, Nascimento Jdos S, Fagundes PC, Nes IF, et al. Aureocins 4185, bacteriocins produced by Staphylococcus aureus 4185: potential application in food preservation. Foodborne pathogens and disease. 2010;7(10):1255–62. Epub 2010/07/14. 10.1089/fpd.2010.0578 . [DOI] [PubMed] [Google Scholar]
- 40.Iwatani S, Zendo T, Yoneyama F, Nakayama J, Sonomoto K. Characterization and Structure Analysis of a Novel Bacteriocin, Lacticin Z, Produced by Lactococcus lactis QU 14. Bioscience, Biotechnology, and Biochemistry. 2007;71(8):1984–92. 10.1271/bbb.70169 [DOI] [PubMed] [Google Scholar]
- 41.Fujita K, Ichimasa S, Zendo T, Koga S, Yoneyama F, Nakayama J, et al. Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Applied and environmental microbiology. 2007;73(9):2871–7. 10.1128/AEM.02286-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandiford S, Upton M. Identification, characterization, and recombinant expression of epidermicin NI01, a novel unmodified bacteriocin produced by Staphylococcus epidermidis that displays potent activity against Staphylococci. Antimicrobial agents and chemotherapy. 2012;56(3):1539–47. 10.1128/AAC.05397-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez RH, Zendo T, Sonomoto K. Circular and Leaderless Bacteriocins: Biosynthesis, Mode of Action, Applications, and Prospects. Frontiers in microbiology. 2018;9:2085–. 10.3389/fmicb.2018.02085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwatani S, Yoneyama F, Miyashita S, Zendo T, Nakayama J, Sonomoto K. Identification of the genes involved in the secretion and self-immunity of lacticin Q, an unmodified leaderless bacteriocin from Lactococcus lactis QU 5. Microbiology. 2012;158(Pt_12):2927–35. 10.1099/mic.0.062943-0 [DOI] [PubMed] [Google Scholar]
- 45.Iwatani S, Horikiri Y, Zendo T, Nakayama J, Sonomoto K. Bifunctional Gene Cluster lnqBCDEF Mediates Bacteriocin Production and Immunity with Differential Genetic Requirements. Applied and Environmental Microbiology. 2013;79(7):2446–9. 10.1128/AEM.03783-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nascimento JdS Coelho MLV, Ceotto H, Potter A, Fleming LR, Salehian Z, et al. Genes Involved in Immunity to and Secretion of Aureocin A53, an Atypical Class II Bacteriocin Produced by Staphylococcus aureus A53. Journal of Bacteriology. 2012;194(4):875–83. 10.1128/JB.06203-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Netz DJA, Sahl H-G, Marcolino R, dos Santos Nascimento Jn, de Oliveira SS, Soares MB, et al. Molecular characterisation of aureocin A70, a multi-peptide bacteriocin isolated from Staphylococcus aureus 11 Edited by M. Yaniv. Journal of Molecular Biology. 2001;311(5):939–49. 10.1006/jmbi.2001.4885 [DOI] [PubMed] [Google Scholar]
- 48.Coelho MLV, Coutinho BG, Cabral da Silva Santos O, Nes IF, Bastos MdCdF. Immunity to the Staphylococcus aureus leaderless four-peptide bacteriocin aureocin A70 is conferred by AurI, an integral membrane protein. Research in Microbiology. 2014;165(1):50–9. 10.1016/j.resmic.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 49.Acedo JZ, van Belkum MJ, Lohans CT, Towle KM, Miskolzie M, Vederas JC. Nuclear Magnetic Resonance Solution Structures of Lacticin Q and Aureocin A53 Reveal a Structural Motif Conserved among Leaderless Bacteriocins with Broad-Spectrum Activity. Biochemistry. 2016;55(4):733–42. Epub 2016/01/16. 10.1021/acs.biochem.5b01306 . [DOI] [PubMed] [Google Scholar]
- 50.Wladyka B, Wielebska K, Wloka M, Bochenska O, Dubin G, Dubin A, et al. Isolation, biochemical characterization, and cloning of a bacteriocin from the poultry-associated Staphylococcus aureus strain CH-91. Applied Microbiology and Biotechnology. 2013;97(16):7229–39. 10.1007/s00253-012-4578-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Towle KM, Vederas JC. Structural features of many circular and leaderless bacteriocins are similar to those in saposins and saposin-like peptides. MedChemComm. 2017;8:276–85. 10.1039/c6md00607h . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoneyama F, Imura Y, Ohno K, Zendo T, Nakayama J, Matsuzaki K, et al. Peptide-lipid huge toroidal pore, a new antimicrobial mechanism mediated by a lactococcal bacteriocin, lacticin Q. Antimicrobial agents and chemotherapy. 2009;53:3211–7. 10.1128/AAC.00209-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M, Yoneyama F, Toshimitsu N, Zendo T, Nakayama J, Sonomoto K. Lethal hydroxyl radical accumulation by a lactococcal bacteriocin, lacticin Q. Antimicrobial agents and chemotherapy. 2013;57(8):3897–902. Epub 2013/06/05. 10.1128/AAC.00638-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Netz DJ, Bastos Mdo C, Sahl HG. Mode of action of the antimicrobial peptide aureocin A53 from Staphylococcus aureus. Appl Environ Microbiol. 2002;68(11):5274–80. Epub 2002/10/31. 10.1128/AEM.68.11.5274-5280.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ovchinnikov KV, Chi H, Mehmeti I, Holo H, Nes IF, Diep DB. Novel group of leaderless multipeptide bacteriocins from Gram-positive bacteria. Applied and Environmental Microbiology. 2016;82:5216–24. 10.1128/AEM.01094-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwatani S, Horikiri Y, Zendo T, Nakayama J, Sonomoto K. Bifunctional Gene Cluster lnqBCDEF Mediates Bacteriocin Production and Immunity with Differential Genetic Requirements. Applied and Environmental Microbiology. 2013;79:2446–9. 10.1128/AEM.03783-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibreel TM, Upton M. Synthetic epidermicin NI01 can protect Galleria mellonella larvae from infection with Staphylococcus aureus. Journal of Antimicrobial Chemotherapy. 2013;68:2269–73. 10.1093/jac/dkt195 [DOI] [PubMed] [Google Scholar]
- 58.Halliwell S, Warn P, Sattar A, Derrick JP, Upton M. A single dose of epidermicin NI01 is sufficient to eradicate MRSA from the nares of cotton rats. The Journal of antimicrobial chemotherapy. 2017;72:778–81. 10.1093/jac/dkw457 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequence of the S. capitis CIT060 genomic region that was analysed in this study was submitted to Genbank (accession number MN234131).