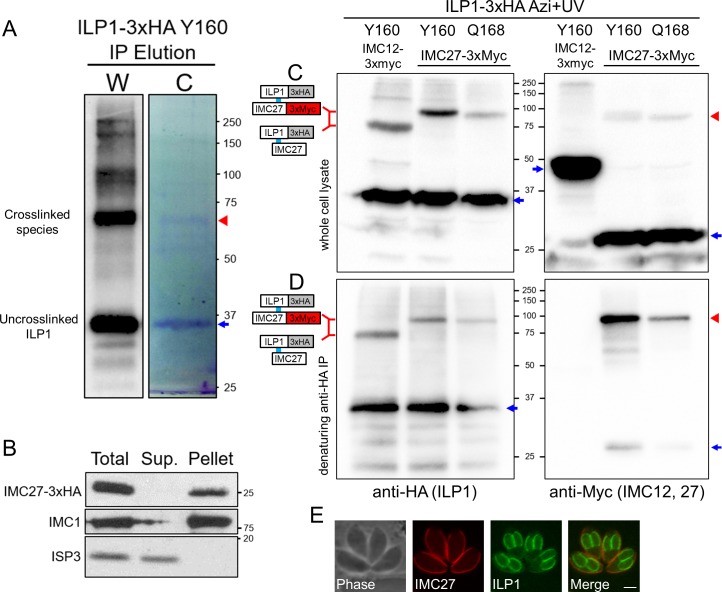
Fig 8. Mass spectrometric identification of crosslinked proteins reveals IMC27 as an ILP1 binding partner.
(A) Western blot (W) and Coomassie gel (C) analyses of large-scale denaturing IP of Y160. The region of gel containing the crosslinked ILP1 species (red arrowhead) was excised and processed for LC-MS/MS peptide identification. Uncrosslinked ILP1 is denoted with a blue arrow. (B) Detergent fractionation showing that IMC27 is firmly associated with the IMC cytoskeleton, like ILP1 [17]. IMC1 is a control for the insoluble fraction whereas membrane-associated ISP3 is readily solubilizes upon detergent extraction. (C) Photocrosslinking of Y160 and Q168 in strains endogenously 3xMyc tagged for IMC27 (or IMC12 as a control). Tagging of IMC27 results in slower migration of the crosslinked product compared to that of the IMC12 tagged strain, indicating IMC27 is the partner. The shifted products are also detected in the anti-Myc blot (second panel, red arrowhead). Uncrosslinked proteins are denoted with blue arrows. (D) Denaturing IP shows the same pattern of shifted products for ILP1. Probing the samples with anti-Myc (for IMC29 or IMC12) confirms the higher migrating shifted product are IMC27 (red arrowhead), while uncrosslinked IMC27 is largely removed (blue arrow). IMC12-3xMyc is completely eliminated by denaturation. Uncrosslinked proteins are denoted with blue arrows. (E) IFA of IMC27-3xMyc parasites reveals that it localizes solely to the maternal IMC, indicating that interaction with ILP1 via Y160 and Q168 occurs within this subcompartment rather than the forming daughters. Red: mouse anti-Myc antibody, green: rat anti-ILP1 antibody. Scale bar represents 2 μm. Azi, p-azidophenylalanine; HA, hemagglutinin; IFA, immunofluorescence assay; ILP1, IMC localizing protein 1; IMC, inner membrane complex; IP, immunoprecipitation; ISP3, IMC subcompartment protein 3; LC-MS/MS, liquid chromatography tandem mass spectrometry.