Fig. 2. Capsid structure of P68.
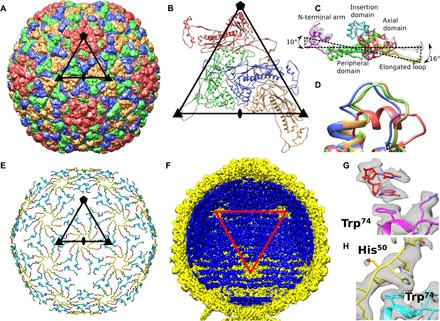
(A) Major capsid proteins of P68 have HK97 fold and form T = 4 icosahedral lattice. Positions of selected icosahedral five-, three-, and twofold symmetry axes are indicated by pentagon, triangles, and oval, respectively. Borders of one icosahedral asymmetric unit are highlighted. (B) Cartoon representation of P68 major capsid proteins in icosahedral asymmetric unit. Positions of icosahedral symmetry axes and borders of icosahedral asymmetric unit are shown. (C) Major capsid proteins from icosahedral asymmetric unit differ in positions of elongated loops and N-terminal domains. Color coding of one of the subunits indicates division of major capsid protein to domains. (D) Residues 253 to 263 from the axial domain of major capsid proteins differ in structure. The residues form an α helix in the subunit that is part of the pentamers, whereas they constitute loops in the other subunits. The color coding of subunits is the same as in (B). (E) The inner capsid protein is organized in a T = 4 icosahedral lattice. Proteins are rainbow-colored from N terminus in blue to C terminus in red. Subunits related by icosahedral threefold axes and quasi-threefold axes of the T = 4 lattice form three-pointed stars in which the C terminus of one subunit is positioned next to the N terminus of another subunit. Borders of a selected icosahedral asymmetric unit are shown. (F) The ordering of the packaged P68 dsDNA genome (shown in blue) is disrupted around the fivefold vertices of the capsid (shown in yellow). Red triangle indicates one face of icosahedron. (G) Stacking interactions of two nucleotides with side chain of Trp74 of major capsid protein located next to fivefold vertex. (H) Side chains of Trp74 of major capsid proteins that form hexamers bind to His50 of inner capsid proteins.
