Fig. 5. HDX-MS suggests allosteric changes in P-Rex1 upon Gβγ binding.
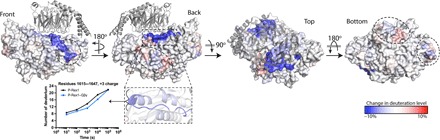
Differences in HDX upon complex formation with Gβγ (at 1000 s) were plotted onto the cryo-EM structure of the P-Rex1 Gβγ-binding module. Red regions, more dynamic behavior upon Gβγ binding; blue regions, less dynamic behavior upon Gβγ binding. Graph shows a comparison of the exchange over time for the indicated structural features. Changes occur distal from the Gβγ-binding site (dashed ovals), suggesting that binding may cause allosteric changes in P-Rex1. These experiments were performed twice, and the data shown represent the average of two experiments. See also data files S1 and S2.
