Fig. 2. Biosynthesis and ultrahigh-throughput screening of a combinatorial cyclic heptapeptide library with expanded diversity for discovering inhibitors of protein aggregation.
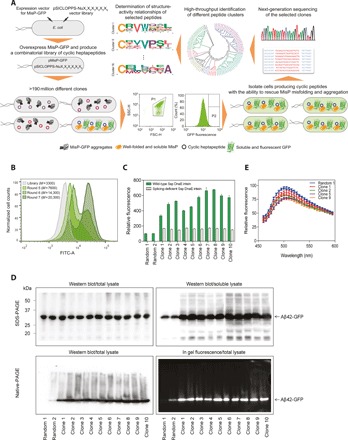
(A) Schematic of the used bacterial platform for discovering inhibitors of protein aggregation and for the high-throughput analysis of the selected hits. pMisP-GFP: plasmid encoding a misfolded protein-GFP fusion; pSICLOPPS-NuX1X2X3X4X5X6: vector library encoding the combinatorial heptapeptide library cyclo-NuX1X2X3X4X5X6; Nu: Cys, Ser, or Thr; X:, any of the 20 natural amino acids; FSC-H: forward scatter; SSC-H: side scatter; P: sorting gate. (B) FACS of E. coli Tuner (DE3) cells overexpressing Aβ42-GFP and the combined cyclic heptapeptide library. M: mean GFP fluorescence in arbitrary units. FITC-A: filter for fluorescein isothiocyanate. (C) Relative fluorescence of E. coli Tuner (DE3) cells overexpressing Aβ42-GFP and 10 randomly selected cyclic heptapeptide clones isolated after the seventh round of FACS shown in (B) and using either the wild-type split Ssp DnaE intein (green bars) or the splicing-deficient variant H24L/F26A (white bars) (25). Two randomly picked cyclic peptide sequences (random 1 and 2) previously shown to have no effect on Αβ42-GFP fluorescence and aggregation (18) were used as a negative control. The fluorescence of the bacterial population producing cyclic peptide random 1 was arbitrarily set to 100. Mean values ± SEM are presented (n = 3 independent experiments, each performed in three replicates). (D) Top: Western blot analysis of total (left) and soluble (right) lysates of E. coli Tuner (DE3) cells overexpressing Aβ42-GFP and the 10 individual cyclic peptide sequences tested in (C). The predicted molecular mass of the Aβ42-GFP fusion is ~32 kDa. Bottom: Western blotting using the anti-Aβ antibody 6E10 (left) and in-gel fluorescence (right) analyses of total lysates following native PAGE of E. coli Tuner (DE3) cells coexpressing Aβ42-GFP and the 10 individual cyclic peptide sequences tested in (C). (E) Emission spectra of E. coli Tuner (DE3) cells overexpressing Aβ42 along with four of the selected cyclic heptapeptide sequences tested in (C) and stained with ThS. The maximum fluorescence of the bacterial population producing cyclic peptide random 1 was arbitrarily set to 100. Mean values ± SEM are presented (n = 1 experiment performed in three replicates).
