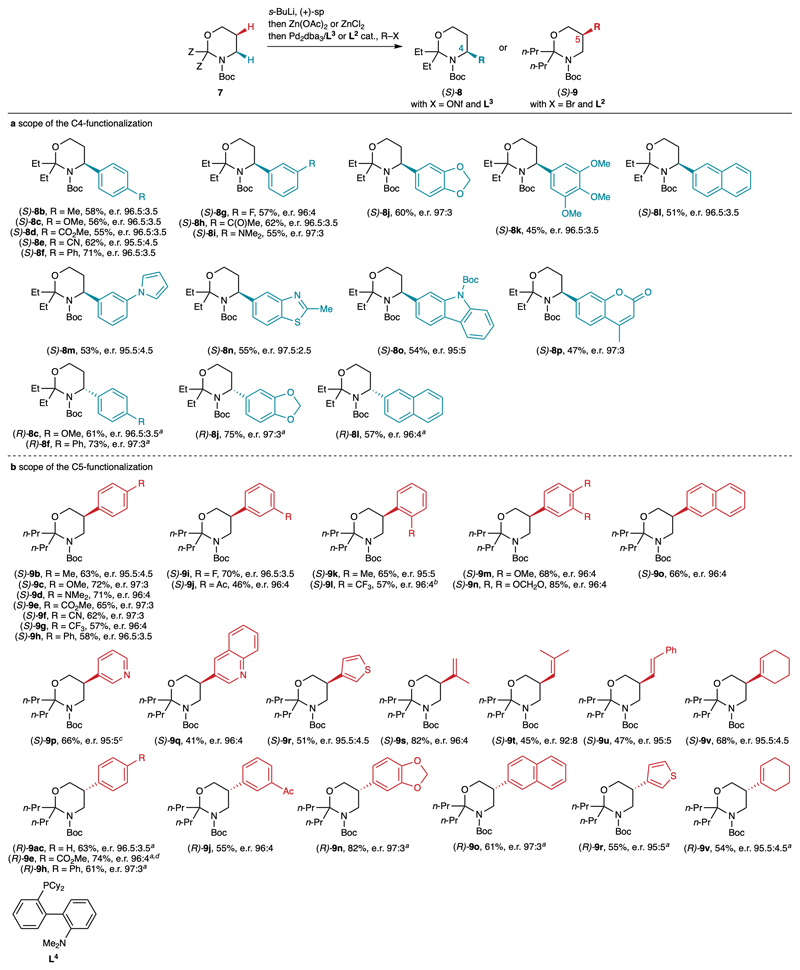
Fig. 4.
Scope of the C4- and C5-functionalization of Boc-1,3-oxazinanes. Reaction conditions: see Table 1, entries 6 (C4-functionalization) and 11 (C5-functionalization). E.r. values were determined by HPLC on a chiral stationary phase, either directly or on the Boc-protected aminoalcohol after cleavage of the aminal. The reference racemic products were synthesized using TMEDA instead of sp. a Using (–)-sp instead of (+)-sp. b Using L4 as the ligand. c Isolated as an inseparable 77:23 mixture of C5- and C6-isomers. d Performed on gram scale; 85% of (–)-sp was recovered.