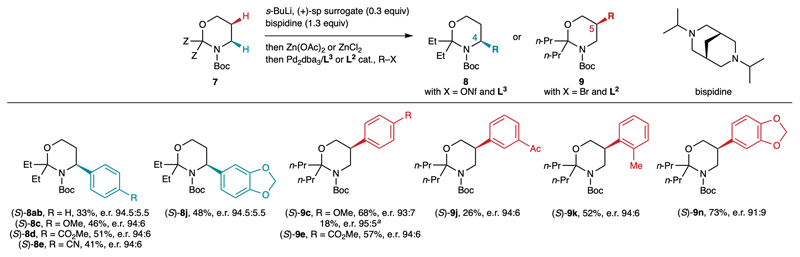
Fig. 5.
Development of proof of concept catalytic enantioselective C4- and C5-arylations. Reaction conditions: 7 (1.0 equiv), s-BuLi (1.2 equiv), (+)-sp surrogate (0.3 equiv), diisopropylbispidine (1.3 equiv), Et2O, –78 °C, 8 h, then Zn(OAc)2 (C4-arylation) or ZnCl2 (C5-arylation), THF (1.2 equiv), –78→20 °C, 1 h, then removal of volatiles, then PhBr (0.7 equiv), Pd2dba3 (2.5 mol%), ligand (5 mol%), toluene, 80 °C, 17 h. a Using (+)-sp instead of the (+)-sp surrogate.