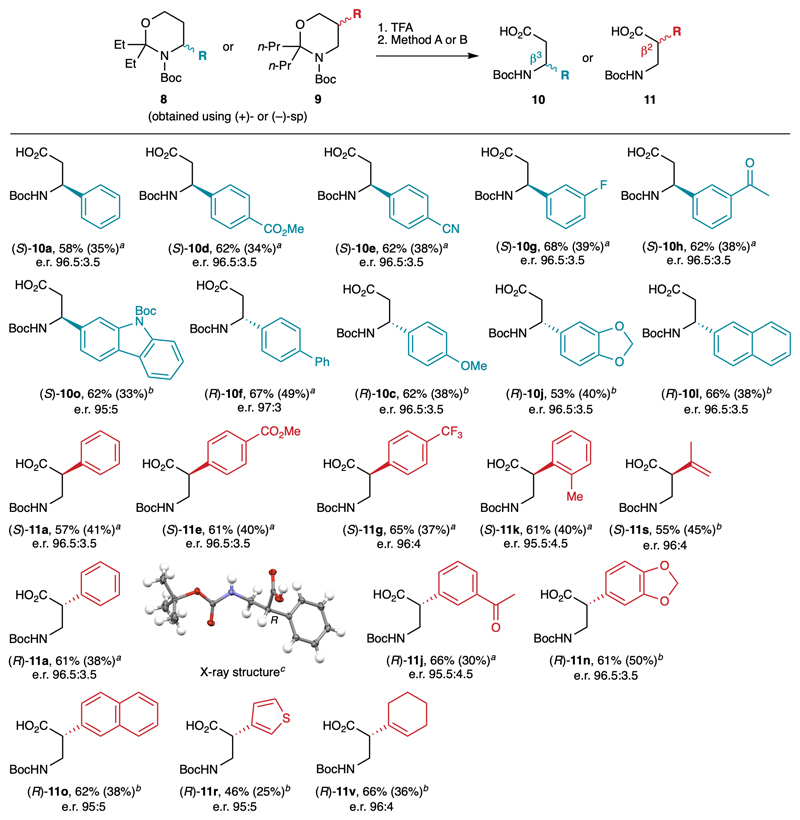
Fig. 6.
Application to the synthesis of β2- and β3-amino acids. Reaction conditions: 1. TFA, THF, 20 °C. 2. Method A: RuCl3 (5 mol%), NaIO4 (3 equiv), MeCN/H2O, 20 °C. Method B: TEMPO (20 mol%), PIDA (2 equiv), CH2Cl2/H2O, 20 °C. (S) enantiomers were obtained with (+)-sp and (R) enantiomers with (–)-sp. Yields in parentheses refer to the overall sequence from Boc-1,3-oxazinanes 7b-c. The e.r. were determined after derivatization to the corresponding methyl esters. a Obtained using method A. b Obtained using method B. c Thermal ellipsoids shown at 50 % probability. TEMPO = (2,2,6,6-tetramethylpiperidin-1-yl)oxyl; PIDA = (diacetoxyiodo)benzene.