Table 1. Effect of selected parameters on the arylation of Boc-1,3-oxazinanes.
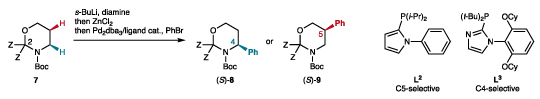 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Z | Reactant | Diamine | Ligand | 8/9a | Product | Yield (%)b | e.r.c |
| 1d | Me | 7a | TMEDA | L3 | >98:2 | 8aa | 54 | – |
| 2d | Me | 7a | (+)-sp | L3 | >98:2 | 8aa | 53 | 75:25 |
| 3d | Et | 7b | (+)-sp | L3 | >98:2 | 8ab | 51 | 95:5 |
| 4d | Et | 7b | (+)-sp surrogate | L3 | >98:2 | 8ab | 46 | 91:9 |
| 5d | n-Pr | 7c | (+)-sp | L3 | >98:2 | 8ac | 30 | 94:6 |
| 6e,f | Et | 7b | (+)-sp | L3 | >98:2 | 8ab | 61 | 97:3 |
| 7a | Me | 7a | TMEDA | L2 | <2:98 | 9aa | 65 | – |
| 8 | Me | 7a | (+)-sp | L2 | <2:98 | 9aa | 51 | 77.5:22.5 |
| 9 | Et | 7b | (+)-sp | L2 | <2:98 | 9ab | 48 | 97:3 |
| 10 | Et | 7b | (+)-sp surrogate | L2 | <2:98 | 9ab | 89 | 94:6 |
| 11f | n-Pr | 7c | (+)-sp | L2 | <2:98 | 9ac | 72 | 96.5:3.5 |
| 12 | –(CH2)4– | 7d | (+)-sp | L2 | <2:98 | 9ad | 23 | 85:15 |
Reaction conditions unless otherwise stated: 7 (1.0 equiv), s-BuLi (1.2 equiv), diamine (1.2 equiv), Et2O, –78 °C, 8 h, then ZnCl2/THF (1.2 equiv), –78→20 °C, 1 h, then removal of volatiles, then PhBr (0.7 equiv), Pd2dba3 (2.5 mol%), ligand (5 mol%), toluene, 80 °C, 17-24 h.
Measured by GCMS or 1H NMR analysis of the crude reaction mixture.
Yield of the isolated product.
Determined by HPLC on a chiral stationary phase.
Cross-coupling step performed at 60 °C instead of 80 °C.
Using Zn(OAc)2 instead of ZnCl2 and PhONf instead of PhBr.
