Figure 6.
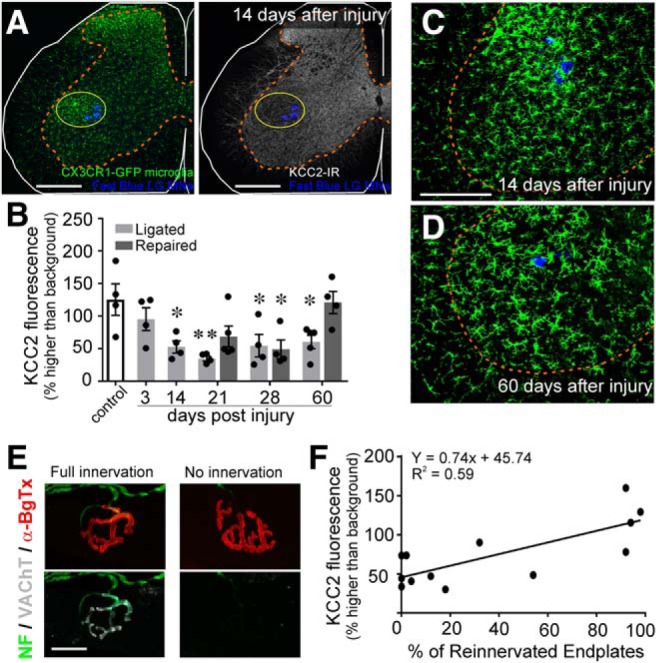
The disappearance of KCC2 coincides with microglial onset but KCC2 restoration depends on muscle reinnervation. CX3CR1EGFP/+ animals underwent unilateral sciatic nerve injury and were allowed to survive for various time points. A, Representative images of microglial (green) and KCC2 (white) IR 14 d after sciatic nerve cut/ligation, at the peak of the microglial response. Scale bars = 200 μm. Regions of KCC2 depletion overlap with the area of microglial reactivity. B, Time course of KCC2 downregulation and recovery in CX3CR1EGFP/+ animals. KCC2 protein levels begin decreasing by 3 d and are significantly reduced by two weeks following injury but are restored to control levels by 60 d in animals in which regeneration was allowed (repaired; Table 4; *p ≤ 0.05, **p ≤ 0.01). Error bars = SEM. C, D, Microglial reaction (green) around injured LG motoneurons (blue) 14 d (C) or 60 d (D) after sciatic nerve injury in cut/ligated animals. KCC2-IR loss appears to coincide with the onset of microgliosis but persists after the response has attenuated. E, Representative motor endplates in the LG muscle following sciatic nerve cut/repair. Acetylcholine receptors were labeled with α-bungarotoxin (red) and motor axons were identified by VAChT (white) and NF-H (green). Scale bars = 5 μm. Endplates with overlapping acetylcholine receptors and VAChT were designated as reinnervated. F, Correlation between KCC2-IR and endplate reinnervation of individual animals (circles). Animals with higher levels of reinnervation of the LG have higher levels of KCC2 fluorescence on LG motoneurons (linear regression; Table 4).
