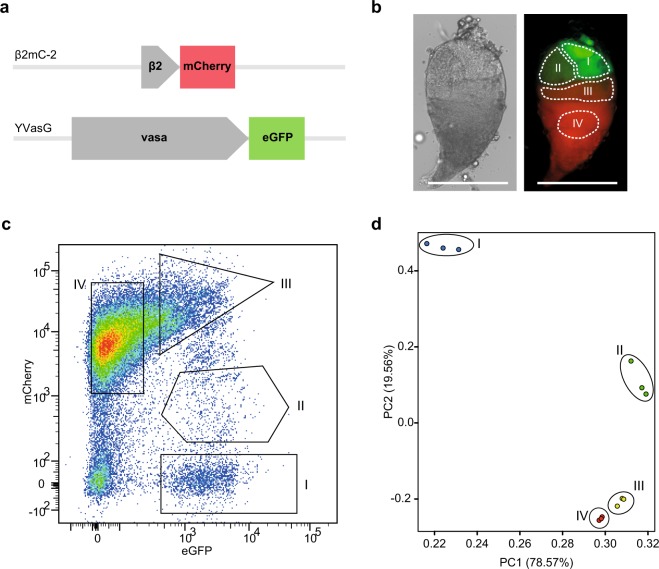
Figure 1.
Isolation and RNA sequencing of germline cell populations from A. gambiae male gonads. (a) Schematic representation of marker transcription units utilised to identify differentiating germline cell populations. The β2:mCherry construct, containing the mCherry fluorescent marker sequence under the transcriptional control of the male meiotic β2 tubulin promoter, was used to generate the β2mC-2 transgenic line. The vasa2:eGFP construct, containing the eGFP sequence under the vasa2 promoter, was previously used to generate the YVasG transgenic line34. The construct used to generate the β2mC transgenic lines also contains piggyBac inverted repeats and a 3xP3 promoter expressing the DsRed marker (3xP3:DsRed) for the selection of single integration events, whilst the YVasG line expresses 3xP3:eCFP (not shown here). β2mC-2 and YVasG transgenic lines were crossed to generate male individuals expressing both β2:mCherry and vasa2:eGFP in the germline. (b) Brightfield and overlay microscopy images showing the distribution of red and green fluorescence in β2mC-2+/YVasG+ dissected testis. Based on combined fluorescence intensity of the red and green marker from each germline cell, four areas (demarcated with dotted lines) were identified as corresponding to the premeiotic (I), meiotic (II and III) and postmeiotic (IV) germ cells. Scale bar: 200 μm. (c) Flow cytometry dot plot of red (mCherry) and green (eGFP) fluorescence (arbitrary units) of live germ cell suspensions obtained from β2mC-2+/YVasG+ testes, displayed using FlowJo biexponential scaling. The four populations were further analysed and separated according to the expression of the transgenic markers, cell size and granularity (Supplementary Fig. S2). (d) Two-dimensional Principal Component Analysis plot from RNA sequencing raw counts showing gene expression clustering of the three independent biological replicates sorted for each cell population.