Figure 6.
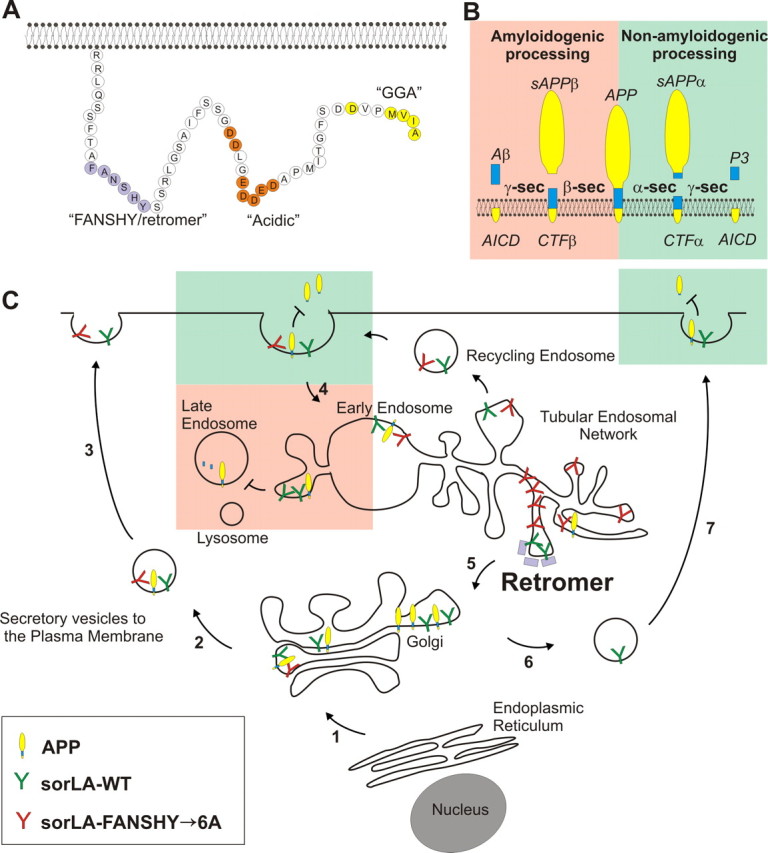
Model of the sorLA intracellular sorting pathways. A, The amino acid sequence of sorLA including known binding sites for the specific cytosolic adaptor proteins retromer (FANSHY), PACS1/AP-2 (acidic sequence), and GGA (MVIA). B, APP processing pathways. In the amyloidogenic pathway (red; to the left), APP is cleaved by β-secretase to generate sAPPβ and subsequent by γ-secretase to produce the Aβ peptide. In the non-amyloidogenic pathway (green; to the right), α-secretase cleaves within the Aβ sequence to generate sAPPα but inhibits amyloid production. C, Schematic roadmap of the major transport pathways of WT and retromer-deficient binding sorLA in cells. Newly synthesized sorLA from ER traverses the Golgi en route to the plasma membrane in the secretory pathway independent of the FANSHY motif (steps 1–3). Both sorLA–WT and sorLA–FANSHY→6A are internalized from the cell surface (step 4) into the early endosome in which the receptors are sorted into distinct tubules of TEN (Andersen et al., 2005; Nielsen et al., 2007; Schmidt et al., 2007). Several pathways exist for exiting the TEN either into recycling endosomes going back to the cell surface or retrograde transport to the Golgi compartment depending on the different cytoplasmic coats surrounding individual tubules. We propose that the sorLA–FANSHY→6A mutant accumulates within SNX-coated tubules, being unable to associate with the cargo-specific VPS subunit of the retromer. In contrast, sorLA–WT is efficiently sorted back to the Golgi (step 5), leading to a higher steady-state level of sorting receptor (compared with the FANSHY→6A mutant) that also leads to higher levels at the cell surface (steps 6 and 7). APP is primarily localized in intracellular compartments, in which the interaction with sorLA–WT retains APP in the perinuclear compartments, leading to less shedding of sAPPα from the cell surface, and hinders entry to the amyloidogenic processing in the late endosomal pathway. sorLA–FANSHY→6A also in part inhibits APP degradation to sAPPα because of the initial localization at the plasma membrane, but there is very little receptor available to inhibit the amyloidogenic sorting into the late endosomes because this variant sorts into the TEN.
