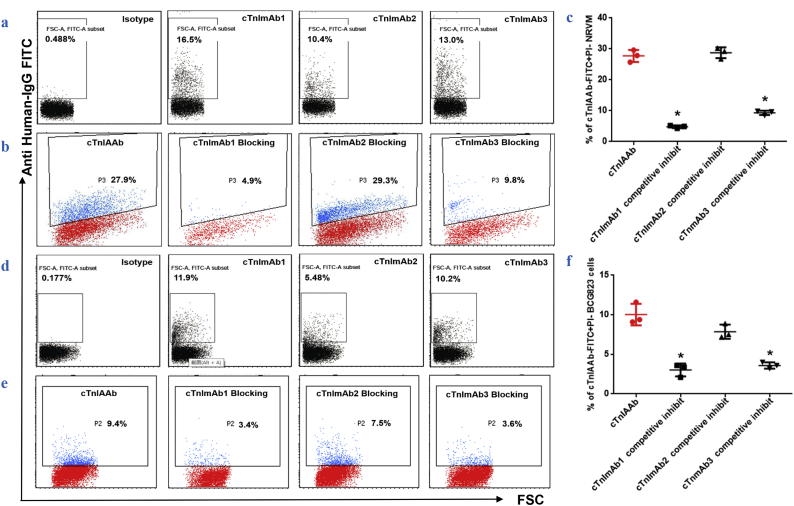
Fig. 3.
cTnImAbs as bioactive alternative for cTnIAAb.
a. Differential binding affinities of cTnImAb1, −2, and −3 for myocardial membrane as revealed by flow cytometry. cTnImAb1 exhibited the highest binding force.
b & c. Flow cytometry showed that cTnImAb1 was a potent competitor against human cTnIAAb in binding to the myocardial cell membrane. Shown are representative images (b) and quantification (c). *p = .000 (ANOVA analysis).
d. Differential binding affinities of cTnImAb1, −2 and −3 for the membranes of BCG823 cells as revealed by flow cytometry. cTnImAb1 exhibited the highest affinity.
e & f. Flow cytometry showed that cTnImAb1 was a potent competitor against human cTnIAAb in binding to the membrane of BCG823 cells. Shown are representative images (e) and quantification (f). *p = .000 (ANOVA analysis).