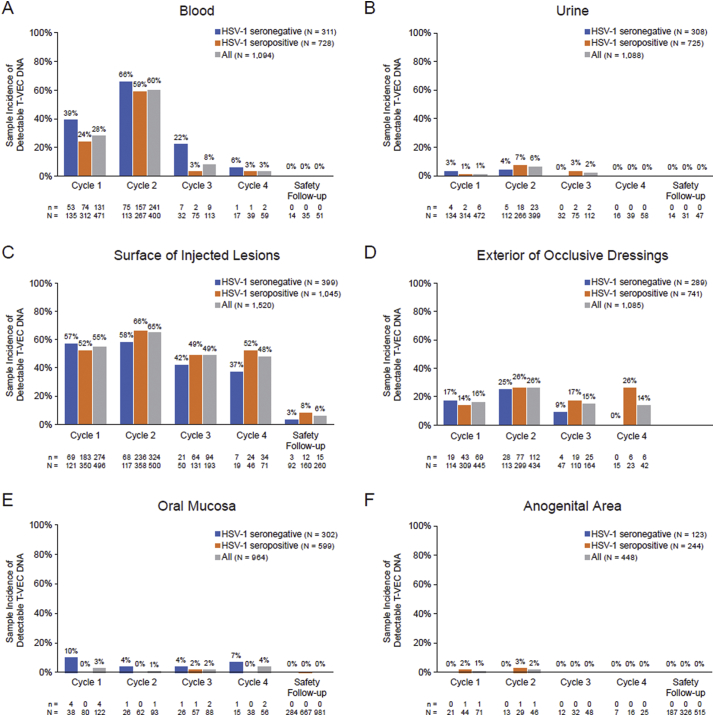
Fig. 3.
Sample incidence of detectable T-VEC DNA in (A) blood, (B) urine, (C) surface of injected lesions, (D) exterior of occlusive dressings, (E) oral mucosa, and (F) anogenital area. HSV-1 serostatus at baseline was missing for 3 patients. Samples were taken on days 30–60 of the safety follow-up period; no swabs from occlusive dressings were taken during the safety follow-up period. A number of samples taken throughout the study contained detectable T-VEC DNA below the lower limit of quantification: 79/383 (21%) samples from blood, 17/31 (55%) samples from urine, 77/741 (10%) swabs from the surface of injected lesions, 52/212 (25%) swabs from the exterior of occlusive dressings, 3/12 (25%) swabs from the oral mucosa, and 5/7 (71%) swabs from the anogenital area. N = number of samples collected; n = number of samples positive for T-VEC DNA. Abbreviations: HSV-1, herpes simplex virus type 1; T-VEC, talimogene laherparepvec.