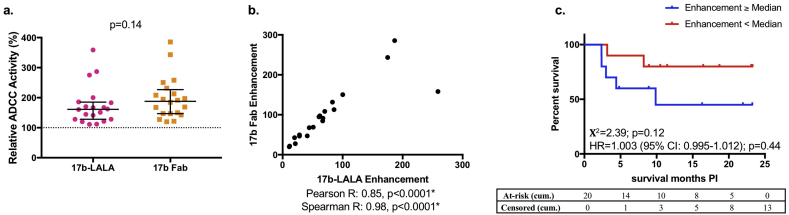
Fig. 5.
Enhancement of plasma ADCC by 17b Fab compared to 17b-LALA.
a: Either 5μg/ml 17b-LALA or 10μg/ml 17b Fab was used as the competitive inhibitor in the competition RFADCC assay with HIV-infected infant plasma (N = 20). Relative ADCC in the presence of 17b-LALA or 17b-Fab is shown and was compared by a Mann-Whitney U test. Error bars represent median + interquartile range. Relative ADCC in the presence of 17b-LALA is averaged from four biological replicates, relative ADCC in the presence of 17b-Fab is averaged from two biological replicates. b: Correlation between enhancement mediated by 17b-LALA and enhancement mediated by 17b-Fab is shown. Pearson and Spearman correlation coefficients and corresponding p-values are shown. c: Kaplan-Meier survival curves between HIV-infected infants (N = 20) that had 17b Fab-mediated passively-acquired ADCC enhancement at/above the HIV-infected infant cohort median (blue lines) or below the HIV-infected infant cohort median (red lines) were compared by a log-rank test. Χ2 values and p-values are shown. The x-axis shows months survival post infection (PI). The association of 17b Fab-mediated enhancement of passively-acquired ADCC with risk of HIV+ infant mortality was measured by a Cox-proportional hazards model. The hazard ratio (HR), 95% confidence intervals (CI), and p-values are shown on the graph. Cumulative (cum.) number of infants at-risk or censored by the end of each month on the x-axis are shown in the tables. Statistical significance was defined as p < 0·05 (*). Data from an individual biological replicate from one infant sample that was below the limit of detection was excluded from the analysis as described in the Materials and Methods. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)