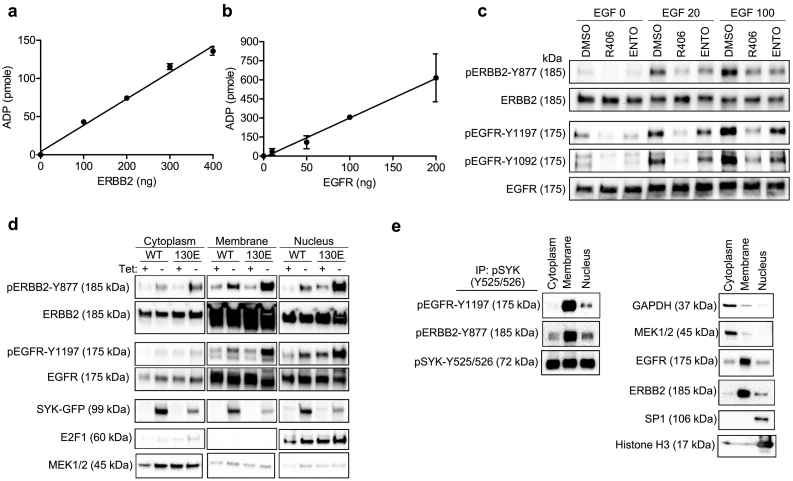
Fig. 1.
SYK phosphorylates and interacts with ERBB2 and EGFR. (A and B) In vitro kinase assay using recombinant active SYK and ERBB2 or EGFR as indicated. ADP-Glo kinase assay was used to measure ADP produced by an in vitro kinase reaction using active recombinant SYK phosphorylation of ERBB2 (A), and recombinant SYK phosphorylation of EGFR protein (B). (C) MPSC1TR cells were serum starved and treated with SYK inhibitors including R406 (700 nM) and entospletinib (ENTO) (700 nM) overnight, and then induced with 0, 20, or 100 ng/mL EGF. The expression of phospho- and total ERBB2 and EGFR were determined by Western blotting. (D) SKOV3 cells ectopically expressing SYKWT or SYK130E active mutant under the Tet-off inducible system were fractionated, and the cytoplasmic, membrane, and nuclear fractions were analyzed for phospho- and total ERBB2 and EGFR expression using Western blotting. MEK1/2, EGFR, ERBB2, and E2F1 serve as positive controls for cytoplasmic, membrane, and nuclear fraction enrichment. (E) Co-immunoprecipitation of EGFR and ERBB2 from subcellular compartments by anti-pSYK. OVISE cells were fractionated, and cytoplasmic, membrane, and nuclear fractions were immunoprecipitated using pSYK(Y525/526) antibody. GAPDH, MEK1/2, EGFR, ERBB2, SP1 and Histone H3 serve as positive controls for cytoplasmic, membrane, and nuclear fraction enrichment.