Abstract
Background
Studies have demonstrated that microRNAs (miRNAs) have essential roles in biological functions of vascular smooth muscle cells (VSMCs). However, the function and related molecular mechanism of miR-149-5p in VSMCs remains unclear.
Material/Methods
We used MTT assay, Transwell assay, and wound-healing assay to measure the proliferation, invasion, and migration of VSMCs transfected with miR-149-5p mimics or inhibitors, respectively. Bioinformatics tools and luciferase assay were used to validate the relationship between miR-149-5p and histone deacetylase 4 (HDAC4). Rescue experiments were used to confirm the interaction of miR-149-5p and HDAC4 in regulating biological functions in VSMCs.
Results
miR-149-5p was downregulated in PDGF-bb-induced VSMCs. It was also found that miR-149-5p overexpression suppressed proliferation, invasion, and migration of VSMCs, while miR-149-5p knockdown showed the opposite effects. Furthermore, HDAC4 was found to be a potential target of miR-149-5p, which rescued miR-149-5p-mediated proliferation, invasion, and migration in VSMCs.
Conclusions
We demonstrated that miR-149-5p can suppress biological functions of VSMCs by regulating HDAC4, which might provide a potent therapeutic target for VSMC growth-related diseases.
MeSH Keywords: Cell Proliferation; Human Migration; MicroRNAs; Models, Cardiovascular
Background
Vascular smooth muscle cells (VSMCs) are the major component of arteries, veins, and microvessels, and with a key role in maintaining vascular structure. When there is pathophysiological vascular remodeling after endothelial dysfunction or damage, they will manage their ability to migrate to the intima and proliferate to supplement neointimal lesions [1]. Primary culture of VSMCs is a pivotal in vitro model, which reserves a high degree of plasticity and can be used to study molecular mechanisms related to a specific physiological or pathological response at the cellular level, ranging from contraction to proliferative [2]. Proliferation and migration of VSMCs lead to medial thickening, resulting in structural remodeling, which has a significant role on the processes of hypertension development. Arterial injury stimulates remodeling responses that, when excessive, lead to stenosis [3]. These responses are influenced by miRNAs signaling in vascular smooth muscle cells and other signaling pathways [3].
MicroRNAs (miRNAs) are a family of endogenous, small, non-coding, single-stranded RNAs about 22 nucleotides in length. They bind to the 3′ untranslated region (3′UTR) of target genes and negatively and post-transcriptionally regulate gene expression by degrading and/or preventing translation of their cognate mRNA target [4,5]. miRNAs play key roles in many cellular processes, such as proliferation [6–8], differentiation [9,10], migration [8,11], and apoptosis [8,12]. miR-149-5p, also referred to as miR-149, was found to be consistently downregulated in acute cellular rejection that occurred in the process of acute cardiac and renal allograft [13]. Further investigations discovered that miR-149 could endure mitochondrial fission and apoptosis by targeting the pro-apoptotic factor p53-upregulated modulator of apoptosis [14]. miR-149-5p was also found to be a functional molecule by affecting cell proliferation [15]. miR-149 exposed on the vascular wall is not only important for heart and vascular development, but is also critical in cardiovascular pathophysiology such as in myocardial infarction or stroke [16,17].
Histone deacetylase 4 (HDAC4), a member of the HDACs family, with an extended N-terminal regulatory domain and a C-terminal tail, is as a crucial manager of cell growth [18], proliferation [19], differentiation [20], and migration [19,21] in various cell types. HDACs performs a deacetylation process that removes acetyl-groups from hyperacetylated histone, which modifies the nucleosome structure, leading to transcriptional silencing [20]. Recent research revealed that downregulation of HDAC4 inhibits the proliferation of VSMCs and reduces vascular calcification [22]. However, the exact mechanism by which HDACs and miRNA are associated is still poorly understood.
Based on the information above, we explored the effect of miR-149-5p on regulating proliferation, invasion, and migration of VSMCs. We found that HDAC4 expression is inhibited by miR-149-5p, which may contribute to biological functions in VSMCs, potentially providing a potent therapeutic target for VSMC growth-related diseases.
Material and Methods
Cell culture and transfection
The VSMCs and HEK293T were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China) and were cultured in Vascular Cell Basal Medium (SmBM, Gibco, USA) supplemented with growth factors and 5% FBS (Gibco, USA), according to the manufacturer’s instructions. Platelet-derived growth factor bb (PDGF-bb, Promega, USA) was added at 20 ng/mL concentration.
For cell transfection, the miR-149-5p mimics, miR-149-5p inhibitor, scramble, and pcDNA3.1-HDAC4 was all obtained from Ribobio (Guangzhou, China). The 50-nM miR-149-5p mimics or inhibitor and 50-nM pcDNA3.1-HDAC4 were transfected into VSMCs using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. After 48-h transfection, the cells were harvested for further analysis.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated using a miRVana kit (Ambion, Belgium). Then, we controlled its integrity and concentration using Nanodrop (Thermo Scientific, Belgium). Synthesis of the first strand (cDNA) was performed using oligo (dT) 20 and Superscript II reverse transcriptase (Thermo Scientific, Belgium). Real-time quantitative PCR was performed using the SYBR green mix (Applied Biosystems, Belgium) on a LightCycler® Nano System (Roche, Switzerland). U6 and GAPDH was used as a housekeeping gene for miRNAs or mRNAs. The sequence of primers for qRT-PCR is detailed in Table 1. The results were analyzed using the relative standard curve of 2−ΔΔCt method.
Table 1.
List of primers used for real-time polymerase chain reaction.
| Name of genes | Sequence (5′-3′) |
|---|---|
| miR-149-5p | Fwd: CCCTCATTCTGTGCCACACTCCAGCTGGG |
| Rev: TGGTGTCGTGGAGTCG | |
| HDAC4 | Fwd: CGCACAGTCCTTGGTTGGT |
| Rev: CTGCTGGAACTGCTGCTT | |
| PCNA | Fwd: GTGCAG ATAATGACAAG |
| Rev: GATTTGACGGCTCCTCT | |
| U6 | Fwd: CACTG GGTGC GGCAG GT |
| Rev: TCATC ACCGA TCGA TACGA TGA | |
| GAPDH | Fwd: CACTCACGGCAAATTCAACGGCA |
| Rev: GACTCCACGACATACTCAGCAC |
Western blot
Total cell lysate was prepared in RIPA lysis buffer (Cell Signaling Technology, USA) and concentrations of proteins were measured using the Pierce BCA Protein Assay kit (Thermo Scientific, Belgium) according to the manufacturer’s instructions. Then, the protein was applied to SDS-PAGE and PVDF membrane (Millipore, USA), then blocked with 5% milk in TBST buffer (Thermo Scientific, USA). Next, we incubated the primary antibodies: anti-PCNA (1: 1000, Abcam UK) and anti-HDAC4 (1: 1000, Abcam UK) or anti-GAPDH (1: 2000, Abcam UK) overnight at 4°C. Then, secondary antibodies (1: 5,000, Abcam UK) were subsequently add to the membranes. Finally, chemiluminescent detection was performed using the ECL system (Millipore, MA, USA).
Cell proliferation assay
The cell proliferation was measured using MTT assay (Sigma, USA). Briefly, 5×103 transfected VSMCs were seeded in 96-well plates, followed by addition of 10 μL MTT Solution and then incubated at 37°C for 4 h. The cell viability was tested by a Benchmark microplate spectrometer (Bio-Rad, USA) using 450 nm absorbance.
Cell invasion assay
Cell invasion was measured using 6-well insert Transwell chambers (BD Biosciences, USA). The transfected VSMCs (3×104/per well) were first suspended in serum-free medium and then add to the upper chamber, while the bottom chamber contained 10% FBS. After 24-h incubation, the cells attached to the upper chamber was removed gently using a sterile cotton swab, and the cells adhered to the insert membrane of the bottom chamber were fixed in 95% ethanol and stained with 0.1% crystal violet. The number of invaded cells were counted in 5 randomly selected fields under a light microscope.
Wound-healing assay
The wound-healing assay was conducted to measure migration rate of VSMCs, as previously reported [23]. The transfected VSMCs (5×104/per well) were seeded into 6-well plates. After 48-h incubation, parallel wounds with similar widths (<3 mm) were made in each well using a 200-ul micropipette tip. Subsequently, wound closure rates were monitored by photographing after culturing for 24 h.
Luciferase assay
Luciferase assays were performed in HEK293 cells. Cells were transfected with each of the plasmids (HDAC4 3′UTR wild-type [WT], and HDAC4 3′UTR mutant [MUT] at miRNA-149-5p binding site) together with miRNA-149-5p mimics and scramble in 24-well plates. After 48-h transfection, luciferase assays were performed by using a luciferase assay kit (Promega, USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (SPSS, Inc., USA). Results are expressed as the mean ±SD. Statistical analysis was conducted by t test or one-way ANOVA. P values less than 0.05 (p<0.05) were considered statistically significant.
Results
miR-149-5p is downregulated in PDGF-bb-induced VSMCs
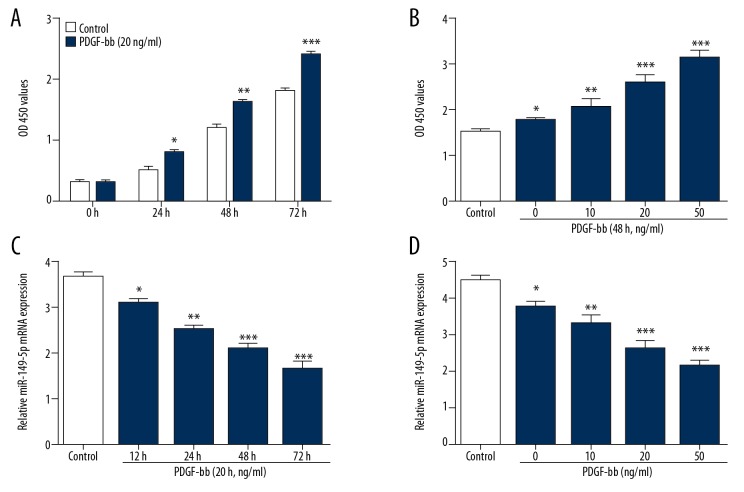
To examine whether miR-149-5p expression was changed in PDGF-bb-induced VSMCs, we measured cell viability of VSMCs stimulated with PDGF-bb at different doses and action times. Figure 1A, 1B shows that PDGF-bb promoted the proliferation of VSMCs in a time-dependent and dose-dependent manner. Moreover, we found that miR-149-5p was downregulated in PDGF-bb-induced VSMCs in a time- and dose-dependent manner (Figure 1C, 1D).
Figure 1.
miR-149-5p is downregulated in PDGF-bb-induced VSMCs. (A, B) The growth ability of VSMCs simulated by different doses of PDGF-bb at various time-points. (C, D) The expression of miR-149-5p in VSMCs simulated by different doses and at time-points of PDGF-bb. * p<0.05 and ** p<0.01 vs. control.
Proliferation of VSMCs is inhibited by miR-149-5p
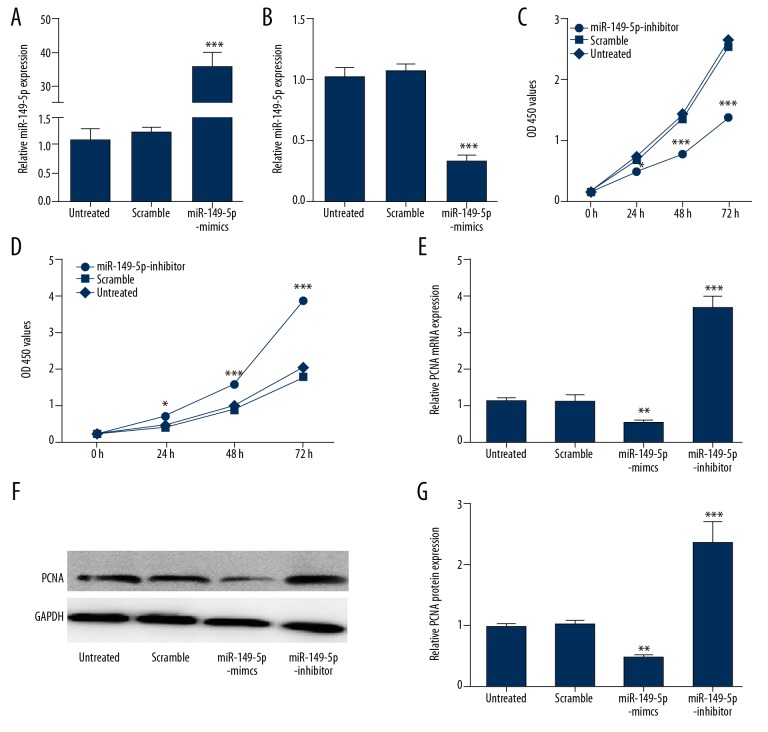
To explore the deep molecular mechanism of miR-149-5p in VSMCs, we constructed loss/gain of function experiments. Our results validated that the expression miR-149-5p was successfully upregulated by miR149-5p mimics and where downregulated by miR-149-5p inhibitor (Figure 2A, 2B). MTT assay showed that proliferation of VSMCs was clearly decreased when transfected with miR-149-5p mimics in comparison with controls (Figure 2C). miR-149-5p inhibitor enhanced the VSMCs proliferation compared with the scramble group and the untreated group (Figure 2C, 2D). Furthermore, we found that miR149-5p overexpression suppressed expression levels of proliferation-related genes such as PCNA in VSMCs, which was dramatically elevated by miR-149-5p inhibitor (Figure 2E–2G), indicating that miR-149-5p had an inhibiting effect on cell growth of VSMCs.
Figure 2.
Proliferation of VSMCs is inhibited by miR-149-5p. (A, B) The overexpression or down-expression of miR-149-5p in VSMCs transfected with miR-149-5p mimics or inhibitor verified by qRT-PCR (C, D). The MTT assay was performed to detect proliferation of VSMCs transfected with miR-149-5p mimics, inhibitor, or control. (E–G) The mRNA and protein level of PCNA in VSMCs transfected with miR-149-5p mimics, inhibitor, or control. * p<0.05 and ** p<0.01 vs. control.
Migration and invasion of VSMCs are suppressed by miR-149-5p
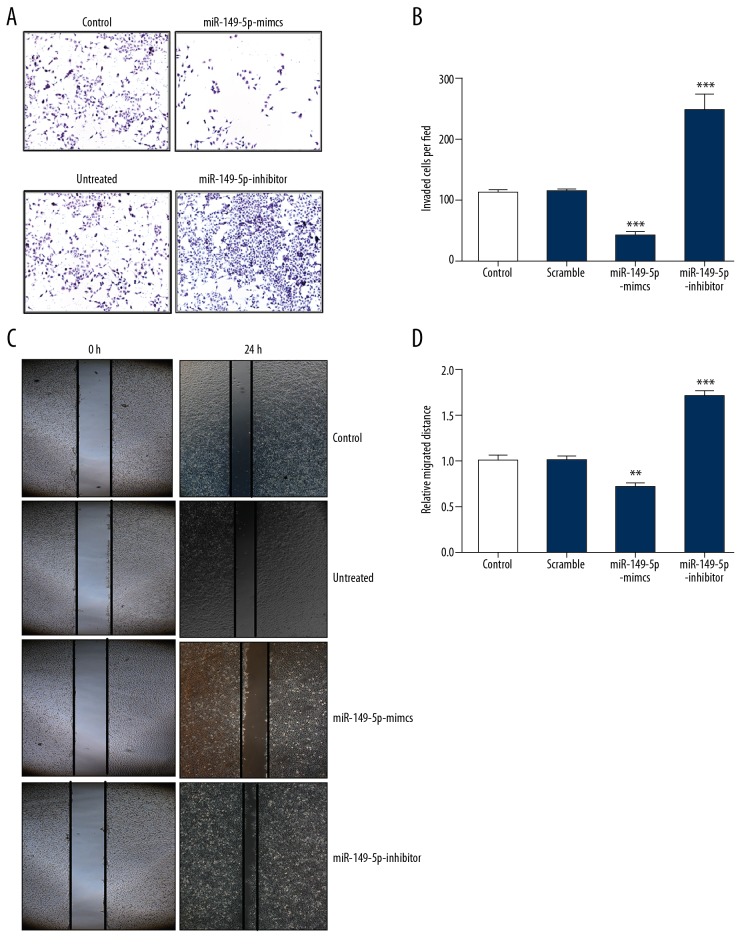
To assess the potential impact of miR-149-5p on migration and invasion in VSMCs, we performed Transwell assay and scratch test, respectively. Our results showed that invasion was significantly repressed in VSMCs transfected with miR-149-5p mimics, but was facilitated by transfection of miR-149-5p inhibitor (Figure 3A, 3B). As shown in Figure 3C, 3D, similar results were found in VSMCs transfected with miR-149-5p mimics, which suppressed migration, while transfection of miR-149-5p inhibitor had the opposite effect. Collectively, our results indicate that miR-149-5p contributes to inhibition of migration and invasion in VSMCs.
Figure 3.
Migration and invasion of VSMCs are suppressed by miR-149-5p. (A, B) Transwell assay was used to evaluate invasion ability of VSMCs transfected with miR-149-5p mimics, inhibitor, or control. (C, D). The cell migration of VSMCs transfected with miR-149-5p mimics, inhibitor, or control was measured by wound-healing assay. * p<0.05 and ** p<0.01 vs. control.
miR-149-5p targets HDAC4 in VSMCs
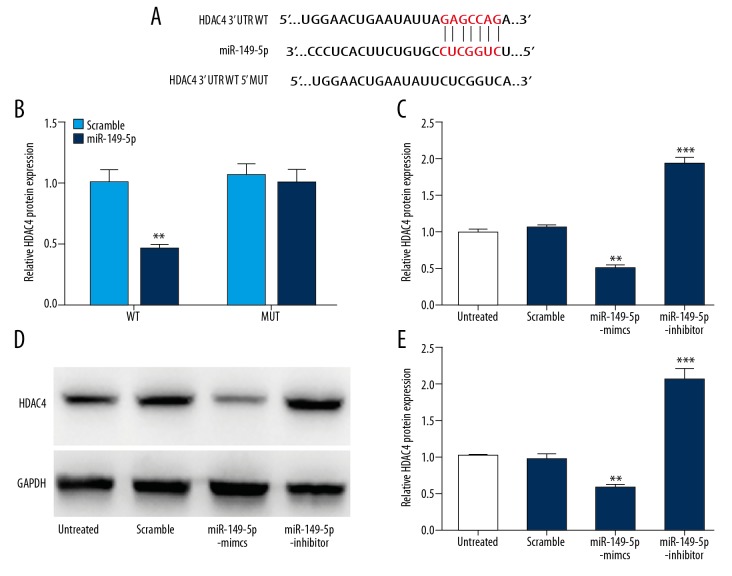
To determine whether miR-149-5p directly regulates HDAC4 through the predicted binding sites in its 3-UTR (Figure 4A), we cloned the 3′UTR region of HDAC4 with the binding site of miR-149-5p into a luciferase reporter and co-transfected it into HEK293 cells with miR-149-5p or controls. As shown in Figure 4B, luciferase assays confirmed that HDAC4 was a target gene of miRNA-149-5p. To further investigate whether HDAC4 is regulated by miR-149-5p, cells were transfected with miR-149-5p mimics or inhibitor and controls into VSMCs, and then we measured the expression of HDAC4 at mRNA and protein levels, respectively. Our results show that miR-149-5p mimics inhibited the expression of HDAC4, while miR-149-5p inhibitor showed the opposite effects (Figure 4C–4E). These data suggest that HDAC4 is a directly target for miRNA-149-5p.
Figure 4.
miR-149-5p targets HDAC4 in VSMCs. (A) Bioinformatics analysis predicted putative miR-149-5p target sites of HDAC4 3′UTR. (B) Luciferase activity of luciferase reporter plasmid containing wild-type or mutant HDAC4 3′UTR co-transfected with miR-149-5p. (C–E) RT-PCR analysis and Western blot were conducted to assess HDAC4 expression in VSMCs transfected with miR-149-5p mimics, inhibitor, or control. * p<0.05 and ** p<0.01 vs. control.
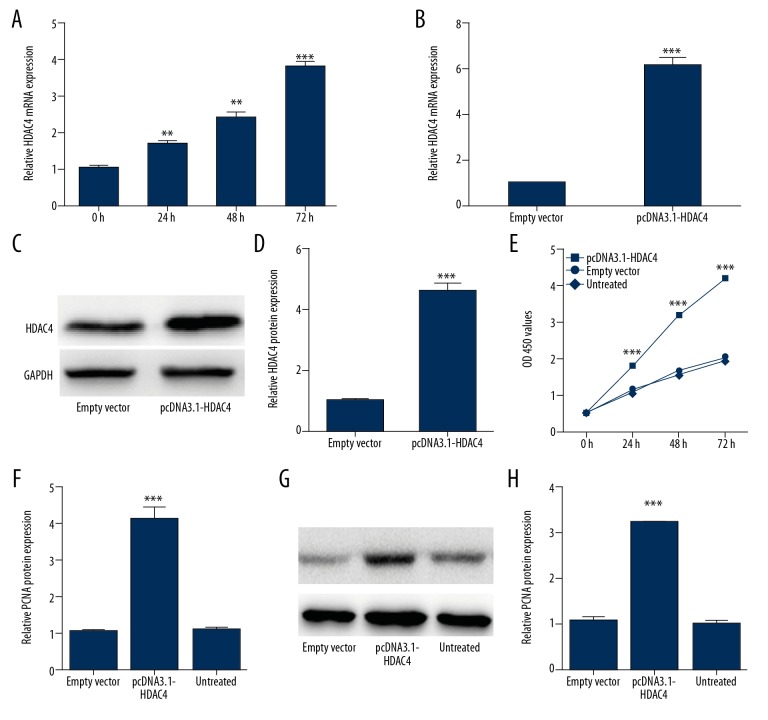
HDAC4 takes part in regulating proliferation of VSMC
To understand the essential role of HDAC4 in VSMC proliferation, we designed a series of related functional experiments. First, mRNA level of HDAC4 was detected in VSMCs after PDGF-bb treatment, showing that PDGF-bb induced HDAC4 expression (Figure 5A). Next, using RT-PCR and Western blot analysis, we verified that pcDNA3.1-HDAC4 activates overexpression of HDAC4 (Figure 5B–5D). Furthermore, our results demonstrated that overexpression of HDAC4 greatly promoted VSMC proliferation as measured by MTT (Figure 5E). Meanwhile, the expression level of PCNA was increased in VSMCs transfected with pcDNA3.1-HDAC4 compared with controls (Figure 5F–5H). All these data suggest that overexpression of HDAC4 strongly promotes VSMCs proliferation.
Figure 5.
HDAC4 takes part in regulating proliferation of VSMC. (A) The expression of HDAC4 in PDGF-bb-stimulated VSMCs was measured by qRT-PCR. (B–D). qRT-PCR and Western blot analysis were were conducted to detect the overexpression efficiency of pcDNA3.1-HDAC4. (E) The proliferation of VSMCs transfected with pcDNA3.1-HDAC4, empty vector, or control were detected by MTT assay. (F–H) The mRNA and protein level of PCNA in VSMCs transfected with miR-149-5p mimics, inhibitor, or control. * p<0.05 and ** p<0.01 vs. control.
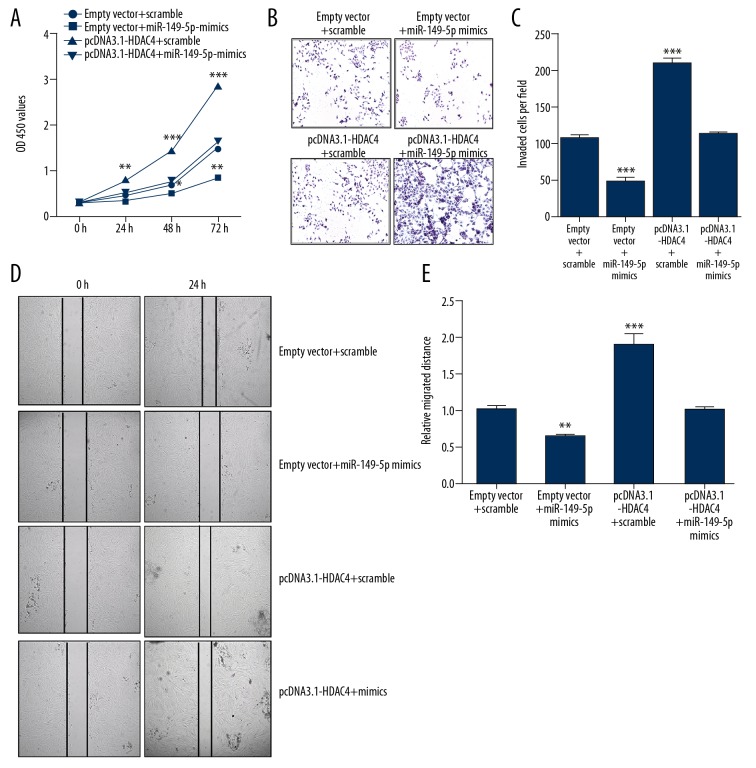
HDAC4 restoration rescues miR-149-5p mediated growth, invasion, and migration
Our previous results indicated that HDAC4 is involved in PDGF-mediated VSMCs proliferation. To further understand whether miR-149-5p and HDAC4 interaction plays a functional role in VSMCs, we used VSMCs co-transfected with either miR-149-5p mimics or scramble and pcDNA3.1-HDAC4 or empty vector. As shown in Figure 6A, the overexpression of HDAC4 promoted VSMCs proliferation, which was rescued by co-transfected miR-149-5p with pcDNA3.1-HDAC4. Moreover, the Transwell assay and scratch text also indicated that overexpression of HDAC4 promotes VSMCs invasion and migration, which was rescued by co-transfection of miR-149-5p with pcDNA3.1-HDAC4 (Figure 6B–6E). Accordingly, we suggest that overexpression of HDAC4 rescues the effect of miR-149-5p on proliferation, invasion, and migration in VSMCs.
Figure 6.
HDAC4 restoration rescued miR-149-5p-mediated growth, invasion, and migration. (A) The proliferation of VSMCs co-transfected with miR-149-5p mimics, pcDNA3.1-HDAC4, or corresponding controls. (B, C) Transwell assay was performed to detect cell invasion in VSMCs co-transfected with miR-149-5p mimics, pcDNA3.1-HDAC4, or corresponding controls. (D, E) Cell migration in VSMCs co-transfected with miR-149-5p mimics, pcDNA3.1-HDAC4, or corresponding controls. * p<0.05 and ** p<0.01 vs. control.
Discussion
Cardiovascular disease has become one of the major causes of death worldwide. Effective therapeutic strategies are limited by poor understanding of the pathogenesis mechanism [1]. Accumulating evidence shows that excessive proliferation and migration of VSMCs plays a role in physiological processes during cardiovascular disease development, and suggests that miRNAs play key roles in regulating some essential steps in progression [24]. Certain miRNAs were found to be suppressive factors for controlling VSMCs proliferation and migration. For example, Xie et al. demonstrated that miR-599 functions as a governor in regulating proliferation and invasion of VSMCs by targeting TGFb2 [25]. Bi et al. reported that miR-503 could modulate VSMC proliferation and migration via suppressing INSR expression [26]. There are far more miRNAs that have the opposite effect of promoting VSMCs proliferation and migration. For instance, Yang et al. showed that miR-541 could facilitate growth ability of VSMCs by regulating IRF7, which might be therapeutic strategy for VSMC growth-related diseases [27]. In our study, we first found that the expression of miR-149-5p was downregulated in PDGF-induced VSMCs in a time- and dose- dependent manner, suggesting that miR-149-5p plays an important role in biological functions of VSMCs.
Recent studies have shown that miR-149-5p is involved in many diseases, including acute myeloid leukemia [28], type 1 diabetes [29], cancer [30–32], and metabolic diseases [13,33], showing the possible mechanism of miR-149-5p-mediated disease progression, including direct cellular function regulation, post-transcriptional control of related genes, and activation of key signaling pathways [28]. For example, Li et al. reported that the expression of miR-149-5p was downregulated in astrocytoma and inhibited cancer cell proliferation and migration, which might serve as a tumor suppressor [34]. In contrast, Fan et al. found that miR-149-5p was abnormally expressed in acute myeloid leukemia, which promotes cancer cell proliferation and arrests the cell cycle and apoptosis, as a result of accelerating tumor progression [15]. Tian et al. also demonstrated that knockdown miR-149-5p induces mitochondrial apoptosis in acute myeloid leukemia cells via regulating FASLG and apoptotic-related genes [28]. However, as a novel miRNA, the function and molecular mechanism of miR-149-5p in VSMCs has not been reported. Our findings show that overexpression of miR-149-5p remarkably suppressed proliferation, invasion, and migration of VSMCs, while knockdown of miR-149-5p showed the opposite effects.
HDAC4 is involved in numerous disease, physiological, and pathological processes [35–37], including stimulating proliferation and migration during neointimal hyperplasia [19,38]. These studies demonstrate that HDAC4 plays a key role in regulation of differentiation and proliferation in many types of cells. HDAC4 also seems to have critical roles in many diseases, including cardiac diseases, cancer, and viral infections [21,36,38]. However, the mechanism by which HDAC4 controls the differentiation and proliferation of VSMCs is not clear. The study of HDAC4 is very important to understand the mechanism of VSMCs differentiation and proliferation and to provide new targets for diagnosis and treatment of hypertension or cardiac diseases. In the present study, HDAC4 was demonstrated to be a direct target of miR-149-5p and participates in PDGF-induced VSMCs proliferation and invasion. We also found that HDAC4 restoration partly rescued miR-149-5p-mediated growth, invasion, and migration in VSMCs, which means miR-149-5p could regulate biological functions in VSMCs by targeting HDAC4.
Conclusions
Our findings suggest a novel role of miR-149-5p in attenuation of PDGF-induced VSMC proliferation, invasion, and migration though inhibition of its specific target, HDAC4. Therefore, we suggest that miR-149-5p might be a therapeutic target in VSMCs-related disease. Further studies are needed to better understand the molecular mechanisms involved in treating miRNA-149-5p-HDAC4-mediated diseases.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Wang G, Jacquet L, Karamariti E, Xu Q. Origin and differentiation of vascular smooth muscle cells. J Physiol. 2015;593(14):3013–30. doi: 10.1113/JP270033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montezano AC, Lopes RA, Neves KB, et al. Isolation and culture of vascular smooth muscle cells from small and large vessels. Methods Mol Biol. 2017;1527:349–54. doi: 10.1007/978-1-4939-6625-7_27. [DOI] [PubMed] [Google Scholar]
- 3.Schlosser A, Pilecki B, Hemstra LE, et al. MFAP4 promotes vascular smooth muscle migration, proliferation and accelerates neointima formation. Arterioscler Thromb Vasc Biol. 2016;36(1):122–33. doi: 10.1161/ATVBAHA.115.306672. [DOI] [PubMed] [Google Scholar]
- 4.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Ann Rev Biochem. 2010;79(1):351–79. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 5.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 6.Xie L, Sadovsky Y. The function of miR-519d in cell migration, invasion, and proliferation suggests a role in early placentation. Placenta. 2016;48:34–37. doi: 10.1016/j.placenta.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DY, Sung JH. Regulatory role of microRNAs in the proliferation and differentiation of adipose-derived stem cells. Histol Histopathol. 2017;32(1):1–10. doi: 10.14670/HH-11-798. [DOI] [PubMed] [Google Scholar]
- 8.Xia W, Zhou J, Luo H, et al. MicroRNA-32 promotes cell proliferation, migration and suppresses apoptosis in breast cancer cells by targeting FBXW7. Cancer Cell Int. 2017;17:14. doi: 10.1186/s12935-017-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu F, Sun B, Xu P, et al. MiR-218 induces neuronal differentiation of ASCs in a temporally sequential manner with fibroblast growth factor by regulation of the Wnt signaling pathway. Sci Rep. 2017;7:39427. doi: 10.1038/srep39427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Shi C, Wang C, et al. The role of miR-497-5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis. Sci Rep. 2017;7:40958. doi: 10.1038/srep40958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JH, Yang R, Zhang W, Wang YP. Functions of microRNA-143 in the apoptosis, invasion and migration of nasopharyngeal carcinoma. Exp Ther Med. 2016;12(6):3749–55. doi: 10.3892/etm.2016.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Mao X, Wang X, et al. miR-433 reduces cell viability and promotes cell apoptosis by regulating MACC1 in colorectal cancer. Oncol Lett. 2017;13(1):81–88. doi: 10.3892/ol.2016.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Aelst LN, Summer G, Li S, et al. RNA profiling in human and murine transplanted hearts: Identification and validation of therapeutic targets for acute cardiac and renal allograft rejection. Am J Transplant. 2016;16(1):99–110. doi: 10.1111/ajt.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding SL, Wang JX, Jiao JQ, et al. A pre-microRNA-149 (miR-149) genetic variation affects miR-149 maturation and its ability to regulate the Puma protein in apoptosis. J Biol Chem. 2013;288(37):26865–77. doi: 10.1074/jbc.M112.440453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan SJ, Li HB, Cui G, et al. miRNA-149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. Leuk Res. 2016;41:62–70. doi: 10.1016/j.leukres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Sung JH, Kim SH, Yang WI, et al. miRNA polymorphisms (miR146a, miR149, miR196a2 and miR499) are associated with the risk of coronary artery disease. Mol Med Rep. 2016;14(3):2328–42. doi: 10.3892/mmr.2016.5495. [DOI] [PubMed] [Google Scholar]
- 17.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118(4):692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren G, Zhang G, Dong Z, et al. Recruitment of HDAC4 by transcription factor YY1 represses HOXB13 to affect cell growth in AR-negative prostate cancers. Int J Biochem Cell Biol. 2009;41(5):1094–101. doi: 10.1016/j.biocel.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Usui T, Morita T, Okada M, Yamawaki H. Histone deacetylase 4 controls neointimal hyperplasia via stimulating proliferation and migration of vascular smooth muscle cells. Hypertension. 2014;63(2):397–403. doi: 10.1161/HYPERTENSIONAHA.113.01843. [DOI] [PubMed] [Google Scholar]
- 20.Huang QK, Qiao HY, Fu MH, et al. MiR-206 attenuates denervation-induced skeletal muscle atrophy in rats through regulation of satellite cell differentiation via TGF-beta1, Smad3, and HDAC4 signaling. Med Sci Monit. 2016;22:1161–70. doi: 10.12659/MSM.897909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Zhou X, Li Q, et al. Role of phosphorylated HDAC4 in stroke-induced angiogenesis. Biomed Res Int. 2017;2017 doi: 10.1155/2017/2957538. 2957538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Wu Z, Xu K, et al. Interfering histone deacetylase 4 inhibits the proliferation of vascular smooth muscle cells via regulating MEG3/miR-125a-5p/IRF1. Cell Adh Migr. 2018;13(1):41–49. doi: 10.1080/19336918.2018.1506653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang CC, Park AY, Guan JL. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protocol. 2007;2(2):329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 24.Seo HH, Kim SW, Lee CY, et al. 7-cyclopentyl-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d] pyrimidin-4-ylamine inhibits the proliferation and migration of vascular smooth muscle cells by suppressing ERK and Akt pathways. Eur J Pharmacol. 2017;798:35–42. doi: 10.1016/j.ejphar.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Xie B, Zhang C, Kang K, Jiang S. miR-599 inhibits vascular smooth muscle cells proliferation and migration by targeting TGFB2. PLoS One. 2015;10(11):e0141512. doi: 10.1371/journal.pone.0141512. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Bi R, Ding F, He Y, et al. miR-503 inhibits platelet-derived growth factor-induced human aortic vascular smooth muscle cell proliferation and migration through targeting the insulin receptor. Biomed Pharmacother. 2016;84:1711–16. doi: 10.1016/j.biopha.2016.10.081. [DOI] [PubMed] [Google Scholar]
- 27.Yang F, Xu Z, Duan S, Luo M. MicroRNA-541 promotes the proliferation of vascular smooth muscle cells by targeting IRF7. Am J Transl Res. 2016;8(2):506–15. [PMC free article] [PubMed] [Google Scholar]
- 28.Tian P, Yan L. Inhibition of microRNA-149-5p induces apoptosis of acute myeloid leukemia cell Line THP-1 by targeting Fas ligand (FASLG) Med Sci Monit. 2016;22:5116–23. doi: 10.12659/MSM.899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grieco FA, Sebastiani G, Juan-Mateu J, et al. MicroRNAs miR-23a-3p, miR-23b-3p, and miR-149-5p regulate the expression of proapoptotic BH3-only proteins DP5 and PUMA in human pancreatic beta-cells. Diabetes. 2017;66(1):100–12. doi: 10.2337/db16-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin l, Li Y, Liu J, et al. Tumor suppressor miR-149-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Mol Med Rep. 2016;13(6):5386–92. doi: 10.3892/mmr.2016.5205. [DOI] [PubMed] [Google Scholar]
- 31.Wei WJ, Lu ZW, Li DS, et al. Association of the miR-149 Rs2292832 polymorphism with papillary thyroid cancer risk and clinicopathologic characteristics in a Chinese population. Int J Mol Sci. 2014;15(11):20968–81. doi: 10.3390/ijms151120968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C, Sun C, Liang X, et al. Integrative analysis of microRNA and mRNA expression profiles in non-small-cell lung cancer. Cancer Gene Ther. 2016;23(4):90–97. doi: 10.1038/cgt.2016.5. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Ni J, Liu YX, et al. Response of MiRNA-22-3p and MiRNA-149-5p to folate deficiency and the differential regulation of MTHFR expression in normal and cancerous human hepatocytes. PLoS One. 2017;12(1):e0168049. doi: 10.1371/journal.pone.0168049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaefer A, Jung M, Mollenkopf HJ, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126(5):1166–76. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 35.Guida N, Laudati G, Mascolo L, et al. p38/Sp1/Sp4/HDAC4/BDNF axis is a novel molecular pathway of the neurotoxic effect of the methylmercury. Front Neurosci. 2017;11:8. doi: 10.3389/fnins.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maddox SA, Kilaru V, Shin J, et al. Estrogen-dependent association of HDAC4 with fear in female mice and women with PTSD. Mol Psychiatry. 2018;23(3):658–65. doi: 10.1038/mp.2016.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, Liu Y, Qin L, et al. Cathepsin H-mediated degradation of HDAC4 for matrix metalloproteinase expression in hepatic stellate cells: implications of epigenetic suppression of matrix metalloproteinases in fibrosis through stabilization of class IIa histone deacetylases. Am J Pathol. 2017;187(4):781–97. doi: 10.1016/j.ajpath.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Zhang X, Yin C, et al. HDAC4 is expressed on multiple T cell lineages but dispensable for their development and function. Oncotarget. 2017;8(11):17562–72. doi: 10.18632/oncotarget.15077. [DOI] [PMC free article] [PubMed] [Google Scholar]