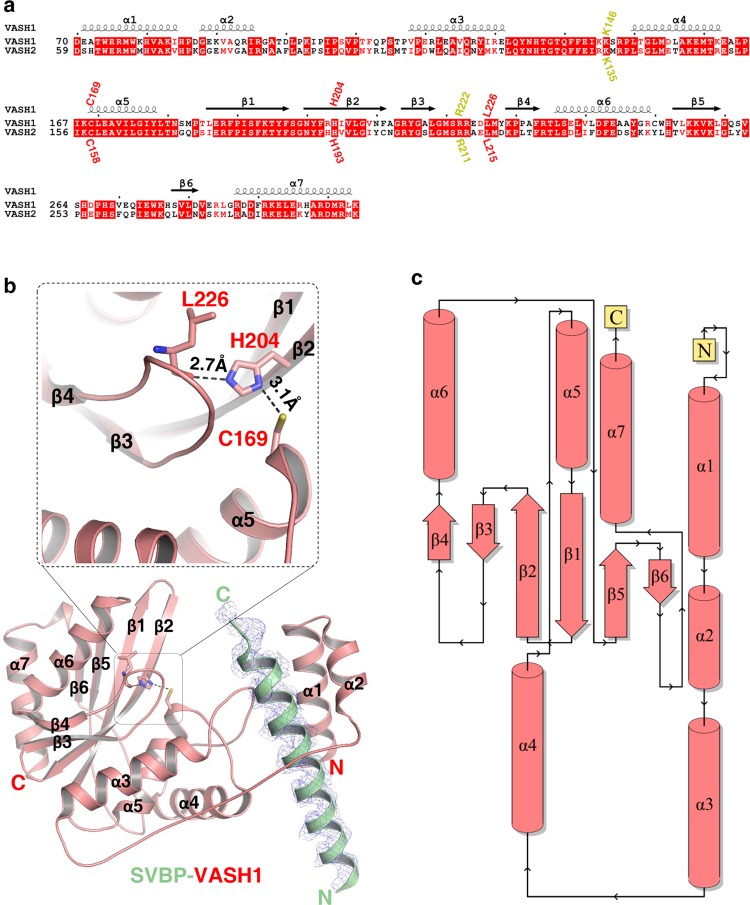
Fig. 1.
Overall structure of the SVBP-VASH1 heterodimer. a Sequence alignment of human VASH1 (NP_055724.1) and VASH2 (NP_001129946.1), with secondary structures labeled at the top of sequences. Catalytic triad of VASH1 (Cys169, His204 and Leu226 of VASH1) and their corresponding residues in VASH2, are marked in red. Lys146 and Arg222 of VASH1 and their corresponding residues in VASH2, are marked in yellow. b Overall structure of the SVBP1–66-VASH170–306 heterodimer. SVBP and VASH1 are shown in green and red cartoon, respectively, with their secondary structures labeled. The catalytic residues of VASH1, including Cys169, His204, and Leu226, are indicated. The |Fo|–|Fc| omit map of SVBP22–50 is contoured at 2.7σ. Hydrogen bonds between His204 and Leu226 and between Cys169 and His204 are indicated by black dashes. c Topology diagram of VASH1 was provided by PDBsum,70 with secondary structures colored in red