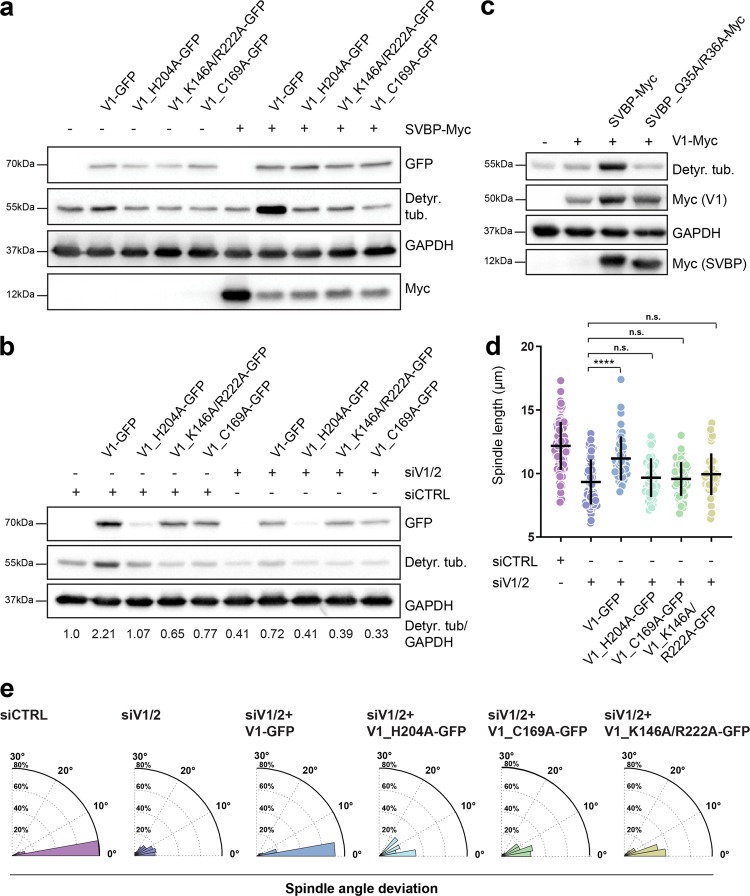
Fig. 6.
Analysis of the mutants linked to the structural features of SVBP-VASH1 complex. a Influence of VASH1 mutations on its activity in the presence and absence of SVBP. Immunoblot of lysates from cells expressing VASH1-GFP mutants with/without SVBP-myc. The activity of VASH1 was assessed with the antibody against detyrosinated tubulin pool. Expression levels of VASH1 and SVBP were observed using anti-GFP and anti-myc antibodies respectively with GAPDH as loading control. b Immunoblotting analysis of detyrosination levels of U2OS cells expressing GFP-tagged VASH1 mutants treated with control or VASH1/2 siRNAs. Expression of VASH1-GFP constructs was detected using anti-GFP antibody, with GAPDH serving as a loading control. Indicated are relative levels of detyrosinated α-tubulin normalized to GAPDH. c Effect of SVBP mutation on activity of SVBP-VASH1 complex was detected by immunoblotting. Levels of detyrosinated tubulin were analyzed in cells overexpressing VASH1-myc with or without overexpression of SVBP-myc constructs. Expression of VASH1 and SVBP constructs were monitored using an anti-myc antibody. d Quantification of the impact of VASH1 activity on preservation of proper spindle length in metaphase U2OS cells treated with control and VASH1/2 siRNAs in presence or absence of VASH1 constructs. N (number of cells, number of independent experiments): siCTRL (94, 6); siVASH1/2 (82, 5); V1 (49, 3); H204A (45, 3); C169A (45, 3); K146A/R222A (45, 3). P-values were calculated using Mann–Whitney U test. n.s.—not significant, ****p < 0.0001. e Polar distribution plots of spindle angle deviation of the same conditions as presented in d