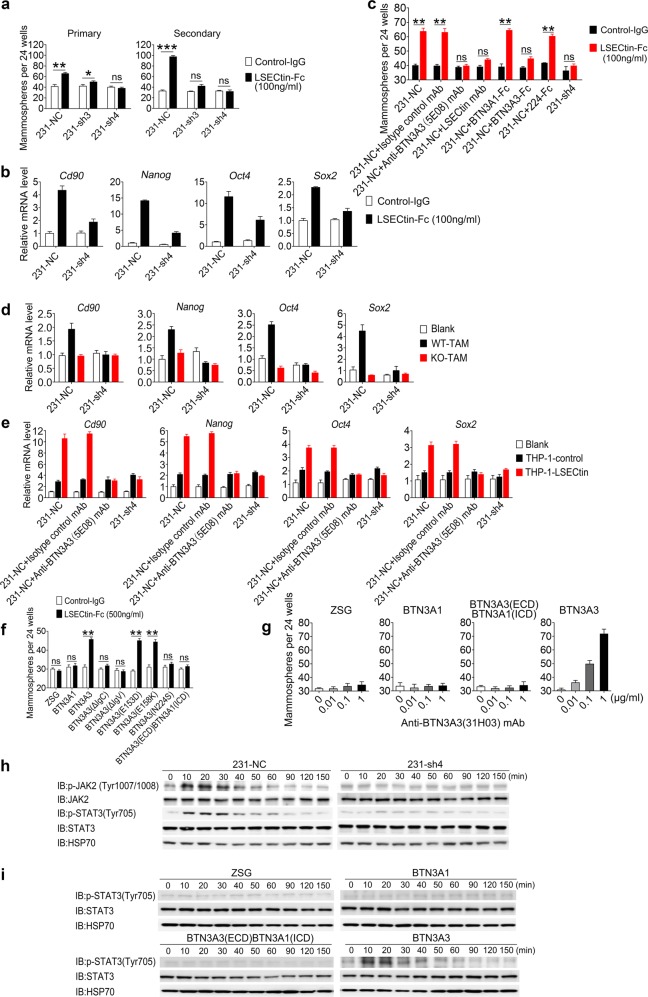
Fig. 4.
The LSECtin-BTN3A3 axis promotes breast cancer stemness in vitro. a Sphere-forming assay showing the representative mean number of spheres of BTN3A3-KD cells (231-sh3 and 231-sh4) or control cells (231-NC) in primary and secondary passaged cultures stimulated with LSECtin-Fc or control-IgG (n = 3 each). One of three experiments is shown. b, d Real-time analysis of the expression of Cd90, Nanog, Oct4 and Sox2 in 231-NC and 231-sh4 mammosphere cultures with LSECtin-Fc or control-IgG (b); with or without primary WT-TAMs or KO-TAMs (d) (n = 3 each). The gene expression shown in Fig. 2e of 231 cells co-cultured with TAMs in a contact fashion is shown for reference. One of three experiments is shown. c Sphere-forming assay showing the representative mean number of spheres. 231-NC cells, 231-sh4 were stimulated with LSECtin-Fc or control-IgG (100 ng/ml). Isotype control mAb, anti-BTN3A3 (5E08) mAb, Anti-LSECtin mAb, BTN3A1-Fc, BTN3A3-Fc or 224-Fc at the same concentration (10 μg/ml) were added to the culture medium (n = 3 each). One of three experiments is shown. e Real-time analysis of the expression of Cd90, Nanog, Oct4 and Sox2 in 231-NC and 231-sh4 mammosphere cultures with or without THP-1-control or THP-1-LSECtin cells (n = 3 each). Anti-BTN3A3 (5E08) mAb or isotype control mAb at the same concentration (10 μg/ml) were added to culture medium to interrupt the LSECtin-BTN3A3 axis. The gene expression shown in Fig. 2f of 231 cells co-cultured with THP-1 cells in a contact fashion is shown for reference. One of three experiments is shown. f, g Sphere-forming assay showing the representative mean number of spheres formed by BT474 cells overexpressing BTN3A3, BTN3A3 deletions, mutants, chimeric proteins or vector in primary passaged cultures stimulated with LSECtin-Fc recombinant protein or control-IgG (f); with anti-BTN3A3 (31H03) mAb in a concentration gradient (g) (n = 4 each). One of three experiments is shown. h 231-NC or 231-sh4 cells stimulated with anti-BTN3A3 (31H03) agonist mAb at 10 μg/ml were lysed and fractionated to obtain cell extracts for WB. JAK, p-JAK, STAT3 and p-STAT3 were immunoblotted to demonstrate signal transduction pathway activation. HSP70 served as a control. i BT474 cells overexpressing vector, BTN3A1, BTN3A3 or BTN3A3 chimeric proteins stimulated with anti-BTN3A3 (31H03) mAb at 10 μg/ml were lysed and fractionated to obtain cell extracts for WB. STAT3 and p-STAT3 were immunoblotted to demonstrate signal transduction pathway activation. HSP70 served as a control. Data are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired Student’s t test for a, c, f). See also Supplementary information, Fig. S4