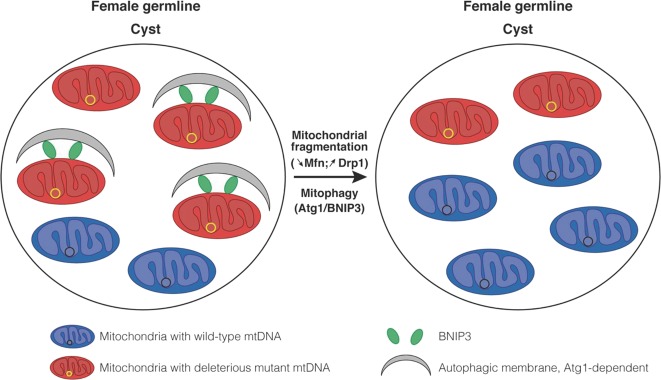
Fig. 1.
Proposed roles of mitochondrial fragmentation and quality control in mutant mtDNA selection in Drosophila germline. Selection of deleterious mutant mtDNA occurs during oocyte development, specifically in the cyst germline. Lieber et al.8 have found that compared to other germ and somatic cells, cyst harbored small, independent and fragmented mitochondria resulting from the lack of the pro-fusion factor Mitofusin (mfn). This mitochondrial size reduction leads to the compartmentalization of mutant mtDNA into smaller organelles, which prevents their mixing with wild-type mtDNA. Mitochondria containing deleterious mtDNA are marked for selection due to a decreased capacity of ATP synthesis. This leads to the mitochondrial recruitment of the mitophagy receptor, BNIP3, and the selective degradation of these mitochondria by autophagy in an Atg1-dependent mechanism, allowing mitochondria containing wild-type mtDNA to re-populate the germline. This new mechanism of mtDNA segregation can be positively or negatively regulated by manipulating key proteins which control either mitochondrial morphology (mfn and Drp1) or quality (BNIP3 and Atg1)