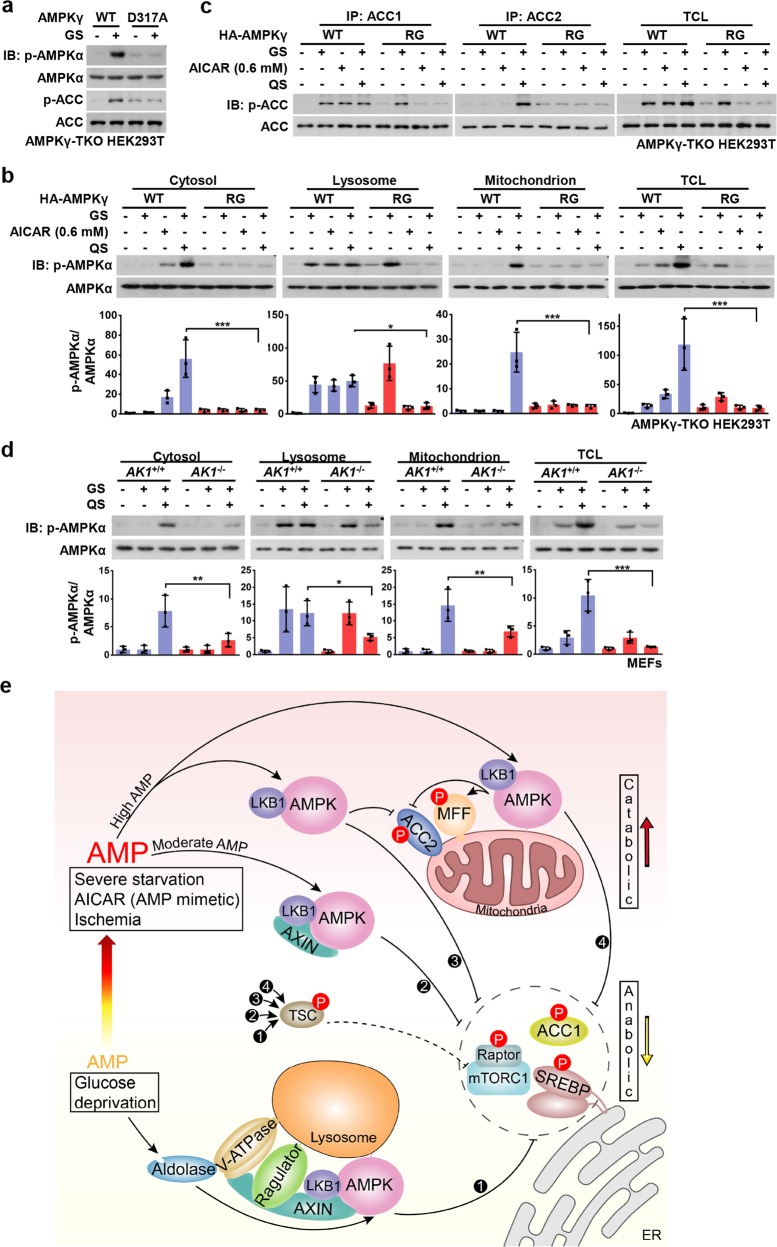
Fig. 4. Roles of AMP in the Hierarchical Activation of AMPK.
a AMPKγ1-D317A impairs glucose-starvation-induced AMPK activation. HA-tagged AMPK-γ1 and its D317A mutant were re-introduced into AMPKγ-TKO HEK293T cells. Cells were then deprived of glucose for 2 h, followed by immunoblotting. b, c AMPKγ2-R531G blocks the phosphorylation of cytosolic and mitochondrial AMPK under moderate and high AMP levels. HA-tagged AMPK-γ2 and its R531G mutant were re-introduced into AMPKγ-TKO HEK293T cells. Cells were then deprived of glucose for 2 h, followed by fractionation and immunoblotting for analyzing p-AMPKα (b), or by immunoprecipitation and immunoblotting for analyzing p-ACC1 and p-ACC2 (c). Statistical analysis data were shown in mean ± SD; ***p < 0.001, *p < 0.05, N.S., not significant by ANOVA, n = 3. d Knockout of AK1 significantly dampens the activation of mitochondrial- and cytosolic-localized AMPK in high AMP conditions. MEFs with AK1 being knocked out were deprived of glucose or both glucose and glutamine for 2 h, followed by fractionation and immunoblotting for analyzing p-AMPKα. Statistical analysis data were shown in mean ± SD; ***p < 0.001, **p < 0.01, *p < 0.05, N.S., not significant by ANOA, n = 3. e A simplified model depicting that the differentially compartmentalized pools of AMPK are activated with different dependencies on AXIN, and the severities of nutrient or energy stress. Glucose starvation, without increase of AMP levels, exclusively activates the lysosomal pool of AMPK through the AXIN-based pathway (➊) which phosphorylates substrates including ACC1, SREBP1, TSC2, Raptor, HDAC4, ULK1, and TBC1D1 to elicit early anti-anabolic roles. Moderately increased AMP levels, during the early phase of severe starvation or after treatment of low concentrations of AICAR, activates cytosolic AMPK, in addition to the lysosomal AMPK (➋), still dependent on AXIN. When AMP levels go up further as a result of severe starvation, ischemia, or treatment of high concentrations of AICAR, cytosolic AMPK (➌) and mitochondrial AMPK (➍) are activated independently of AXIN, leading to phosphorylation of ACC2 and MFF and accelerating catabolic activities, along with all the other substrates that can be phsophorylated at lower AMP levels. Of note, TSC2 and Raptor can be phosphorylated by all the four modes to respectively inhibit mTORC1 activity. Experiments in this figure were performed three times. See also Supplementary information, Fig. S4