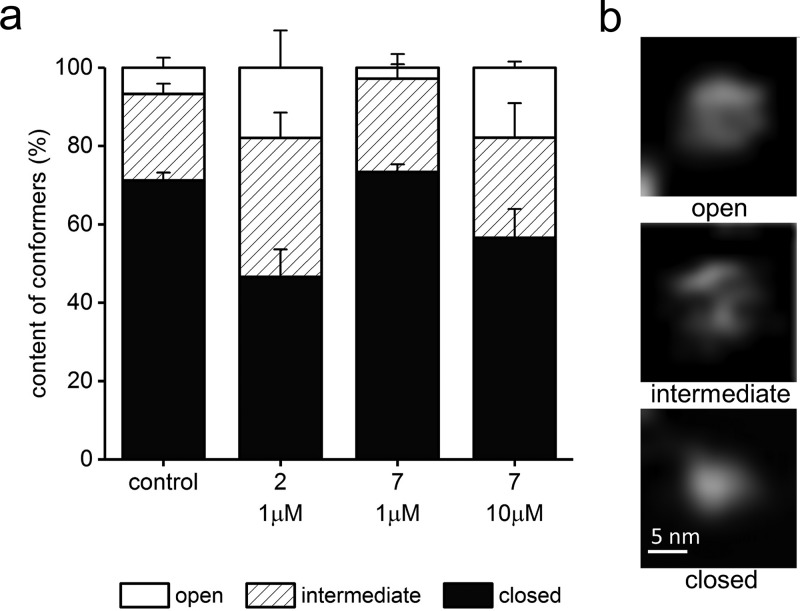
Figure 8.
PR peptides influenced the abundance of the α-face conformations of 20S proteasome, as detected by AFM. (a) In control, untreated samples, about 70% of molecules had their central channel covered and classified as a closed gate conformation. About 7% of molecules had their gate completely open, and the remaining 22% were in the process of switching between these conformations and were classified as intermediates. In the presence of an activating 1 μM concentration of 2, the abundance of open and intermediate conformations increased to 54% (18% and 36%, respectively). In contrast, a 1 μM concentration of 7 decreased the number of open conformers to less than 3%. The higher concentration of 7 pushed proteasome to open the gate in 18% of molecules, with a slightly higher contribution of intermediates (25%). (b) Representative AFM images of the three conformational forms of the core proteasome. The top-view images with the α-face exposed were zoomed-in from 1 μm × 1 μm fields. The images are raw and have been subjected only to planefitting/flattening, linear adjustments of brightness and contrast, and linear interpolation for viewing clarity.