Fig. 1.
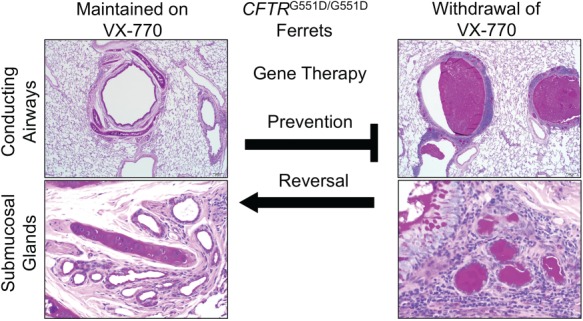
Utilizing CFTRG551D ferrets for testing gene therapies to the CF lung. CFTRG551D/G551D ferrets are protected from lung disease while treated with VX-770. Following termination of VX-770, these CF animals develop mucus accumulation in the airways and submucosal glands and eventually develop lethal bacterial infections of the lung (58). Testing gene therapies in this model can utilize two approaches. Prophylactic gene therapies administered to the lungs of CF ferrets prior to cessation of VX-770 can be used to test whether the gene therapy approach prevents the development of lung disease. This approach circumvents a potential barrier (thick mucus) in the CF airway that may impede gene transfer to the airways and addresses whether the gene therapy approach would be successful in a pristine airway. The more clinically relevant use of this CF ferret model is to ask whether the gene therapy approach can reverse lung disease. In this approach, VX-770 treatment would be terminated prior to gene delivery to the lung. In this scenario, varying extents of lung disease and mucus accumulation could be controlled by the time animals are removed from VX-770 to understand components of disease that are reversible and irreversible. Irreversible components might include structural changes to the lung such as bronchiectasis and bacteria that have adapted to the damaged lung. Comparing the efficacy of both approaches to either prevent or reverse CF lung disease will inform disease specific barriers to gene delivery to the airways and therapeutic obstacles to the approaches tested. Histology panels were reformatted from Supplementary Figure 4 (panels N, P, F and G) in Ref. (58). In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis, Sci. Transl. Med., 11, eaau7531 (Reprinted with permission from AAAS).
