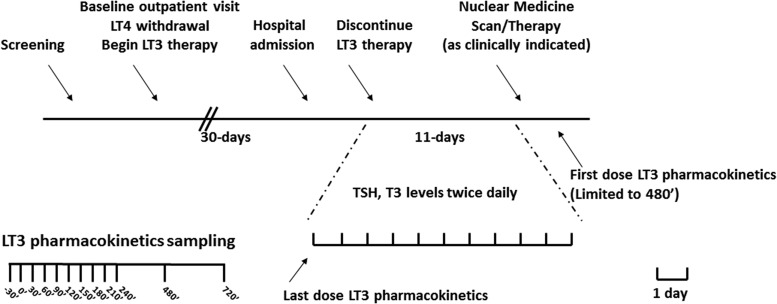
FIG. 1.
Study design. Top panel: timeline of the study. After enrollment, study volunteers were treated with LT3 for at least 30 days before admission to the Clinical Center for last-dose PK and terminal elimination studies. An abbreviated PK study was offered following the nuclear medicine procedures. Bottom panel: time of PK blood sampling. LT3, L-triiodothyronine; LT4, levothyroxine; PK, pharmacokinetics; T3, triiodothyronine; TSH, thyrotropin.