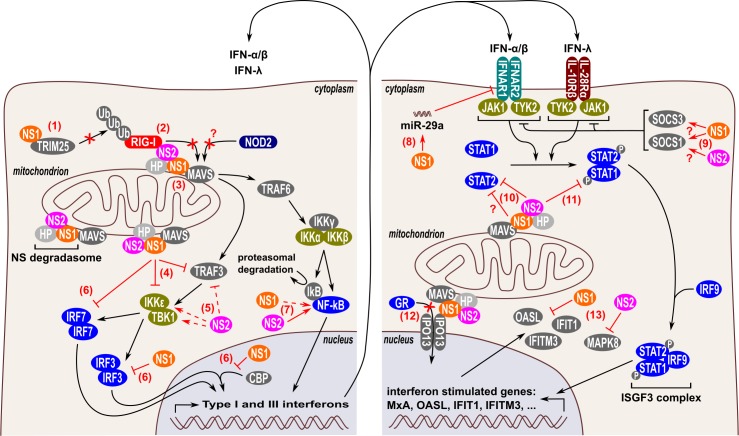
Fig 2. RSV NS1 and NS2 effector functions that can suppress IFN responses.
Type I and III IFN responses are inhibited by NS1 and NS2 at multiple levels, both during the induction of type I and III IFNs (left panel) and during IFN-induced signaling (right panel). NS1 and NS2 can form a so-called “NS degradasome” complex that is stabilized by mitochondria via MAVS. The NSD complex is thought to contain HPs, including the proteasome α2 subunit and other as yet unidentified proteins. NS1 and NS2 prevent the interaction of RIG-I with MAVS in different ways. NS1 binds to the PRY-SPRY domain of TRIM25, which is responsible for the interaction of TRIM25 with RIG-I. As such, NS1 prevents the TRIM25-mediated K63-linked polyubiquitination of RIG-I, which is necessary for the subsequent interaction of RIG-I with MAVS (1). Moreover, NS2 directly interacts with RIG-I (2) and NS1 interacts with MAVS (3) to suppress binding of RIG-I to MAVS. Whether the interaction of NS1 with MAVS also prevents the interaction of NOD2 with MAVS is currently unclear. NS1 reduces protein expression of TRAF3 and IKKε (4), whereas NS2 modestly reduces TRAF3 and induces IKKε and TBK1 (5). NS1 subsequently inhibits IRF3 and IRF7 by different proposed mechanisms (6). NS1 reduces IRF3 and IRF7 protein expression and prevents the interaction between IRF3 and CBP, thereby lowering the binding of the IRF3-CBP complex to the IFN-β promoter. NS2, and to a lesser extent NS1, enhances the activation and nuclear translocation of NF-κB (7). These NS1/NS2 effector functions (1–7) synergistically reduce the production of type I and III IFNs. Furthermore, NS1 and NS2 also suppress type I and III IFN receptor-mediated signal transduction. NS1 induces miR-29a expression, which targets the mRNA coding for IFNAR1, one of the 2 subunits of the type I IFN receptor (8). NS1 and NS2 may induce expression of SOCS proteins (SOCS1 and 3), which negatively regulate the tyrosine kinases JAK1 and TYK2, which are important to transmit signaling from the type I and III IFN receptors (9). NS2 inhibits JAK1/TYK2-mediated activation of STAT1/2 by reducing STAT2 protein levels (10) and by reducing STAT1 phosphorylation (11). Some groups, however, reported that STAT2 expression can also be reduced by NS1 (see text) (10). NS1 and NS2 counteract the anti-inflammatory activity of the GR, although the exact mechanism is debated. In one model, NS1 interacts with the nuclear translocator IPO13, which competes with GR for its nuclear translocation (12). Recent evidence suggests that the NS proteins may also counteract antiviral effector functions of ISGs, e.g., NS1 degrades the OASL, IFIT1, and IFITM3 proteins, whereas NS2 degrades MAPK8 (13). Full and dashed lines indicate robust and moderate inhibitory or stimulatory effector functions, respectively. CBP, CREB binding protein; GR, glucocorticoid receptor; HP, host protein; IFIT, interferon-induced protein with tetratricopeptide repeats; IFITM, interferon-induced transmembrane protein; IFN, interferon; IFNAR, interferon alpha/beta receptor; IKKε, inhibitor of nuclear factor-kappa B kinase subunit epsilon; IPO13, importin-13; IRF3, interferon regulatory factor 3; ISG, IFN-stimulated gene; JAK, janus kinase; MAPK8, mitogen-activated protein kinase 8; MAVS, mitochondrial antiviral-signaling protein; NS, nonstructural; OASL, 2′-5′-oligoadenylate synthase-like protein; RIG-I, retinoic-acid-inducible gene-I; RLR, RIG-I-like receptor; RSV, respiratory syncytial virus; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; TBK1, tank binding kinase 1; TRIM25, tripartite motif–containing protein; TYK, tyrosine kinase 25.