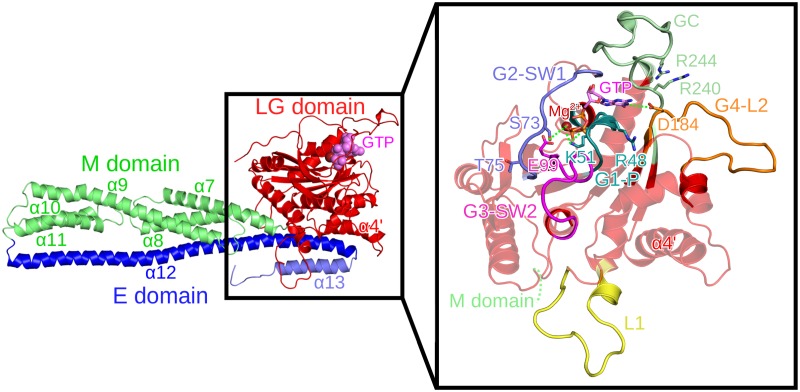
Fig 1. Conformation of the nucleotide-bound hGBP1 crystal structure (PDB 1F5N).
The different domains are highlighted in different colors: the LG domain in red with GTP shown in purple, the M domain in green, and the E domain in blue (with different shades used for α12 and α13 for ease of distinction). Loops missing in the crystal structure were modeled. In the enlargement of the LG domain shown on the right, the four GTP-binding site motifs are displayed: the G1-P loop in turquoise, G2-SW1 in blue, G3-SW2 in magenta, and G4-L2 in orange. The guanine cap (green), loop L1 (yellow), and residues important for dimerization or GTP binding and hydrolysis (shown as sticks with the same color as the corresponding loop) are also highlighted. The dotted green lines indicate direct interactions of hGBP1 residues with GTP and Mg2+.