Summary
Fecal calprotectin has successfully been widely recommended as a sensitive biomarker of inflammatory bowel diseases (IBD). Recently, we have identified an excellent new fecal biomarker, B cell activating factor (BAFF), as being as effective as fecal calprotectin for diagnosing intestinal inflammation. In this study, a total of 230 patients with abdominal discomfort were prospectively enrolled and fecal samples were collected within 24 h before the endoscopic examinations. We show that fecal BAFF levels were significantly higher in patients with ulcerative colitis (median = 1549 pg/g, P < 0·0001), Crohn’s disease (median = 735 pg/g, P < 0·0001), gastric cancer (median = 267 pg/g, P < 0·0001) and colorectal cancer (median = 533 pg/g, P < 0·0001) than those in healthy groups (median = 61 pg/g), while the values of which in patients with gastric polyps, colorectal polyps, esophagitis/gastritis/duodenitis and peptic ulcer were in the range of healthy individuals (P > 0·05). An optimal cut‐off value at 219·5 pg/g of fecal BAFF produced sensitivity, specificity, positive predictive and negative predictive values of 85, 91, 84 and 92%, respectively, for IBD or carcinoma. Our results therefore indicate a potential role for fecal BAFF as a sensitive screening parameter for IBD and gastrointestinal carcinoma, as well a useful tool to select patients with abdominal discomfort for further endoscopic examinations.
Keywords: B cell activating factor, calprotectin, inflammatory bowel diseases, gastrointestinal neoplasms
Introduction
Abdominal discomfort, such as abdominal pain, bloating, diarrhea and rectal bleeding, are frequent reasons for a visit to the gastroenterology clinic. However, those gastrointestinal symptoms are not disease‐specific, which presents great difficulties even for experienced gastroenterologists to diagnose 1, 2. Common clinical diagnostic methods, such as serological, radiological and endoscopic techniques, have their own limitations or flaws. The markers of systemic inflammation such as C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are not sufficiently sensitive and specific for intestinal inflammation and are poorly associated with clinical symptoms and disease activity 3, 4, 5, 6, 7. Radiological examination involves too much exposure to radiation. Endoscopic examination is uncomfortable, invasive and not always available. Meanwhile, it is not reliable to select patients for endoscopy according to clinical symptoms 8, 9, and a relatively large proportion of patients with gastrointestinal symptoms have negative endoscopic results. Thus, non‐invasive fecal biomarkers are increasingly present and it is highly desirable to find surrogate screening biomarkers, which can precisely evaluate the risk stratification of patients with different diagnoses, to effectively reduce unnecessary invasive endoscopies.
The B cell‐activating factor of the tumor necrosis factor (TNF) ligand superfamily (BAFF, also known as TNFSF13B, BLyS, TALL‐1 or THANK), a positive factor for B cell survival, maturation and physiological functions, is predominantly produced by myeloid cells, including monocytes, macrophages, neutrophils and dendritic cells 10, 11, 12, 13. The elevated level of BAFF has been found in various autoimmune diseases, such as systemic lupus erythematosus 14, rheumatoid arthritis 15, 16, 17, multiple sclerosis 18 and primary Sjögren’s syndrome 19, 20. Currently, we have found for the first time that BAFF in serum and feces were elevated in IBD patients, and significant correlations were also elucidated between serum BAFF level and disease activity in ulcerative colitis (UC) patients, indicating that BAFF may be involved in the pathogenesis of inflammatory bowel disease (IBD) 21. Furthermore, we have demonstrated that fecal BAFF correlated well with endoscopic inflammation severity, suggesting fecal BAFF as a promising non‐invasive parameter for discriminating IBD from irritable bowel syndrome (IBS) and for evaluating intestinal inflammation in IBD patients 22.
However, the value of fecal BAFF in unselected patients with abdominal discomfort still needs to be explored. The purpose of our study was therefore to assess the diagnostic value of fecal BAFF compared with fecal calprotectin, an efficient tool for discriminating IBD and IBS, in patients with abdominal discomfort. The potential of measuring BAFF in feces as a method of screening for IBD and gastrointestinal neoplasms was also evaluated.
Materials and methods
Patients
Patients who presented with abdominal discomfort, such as abdominal pain, bloating or altered bowel habit, were prospectively recruited into the study at the Union Hospital and Tongji Hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology from June to August 2016. Abdominal discomfort had to be the chief complaint if several symptoms were presented at the same time 23. Finally, a total of 230 participators were consecutively enrolled. Each recruited patient was instructed to provide a single stool sample using a disposable plastic container within 24 h before esophagogastroduodenoscopy (EGD) or bowel preparation for colonoscopy. Upon receipt, fecal samples were stored at –80ºC and were then analyzed simultaneously.
Patients with concomitant and serious cardiopulmonary, hepatic, renal, neurological, mental and rheumatic disease were specifically excluded from the study; also excluded were patients with failure to collect stool samples or incomplete endoscopy or pregnancy. No patients were included with intake of non‐steroidal anti‐inflammatory drugs (NSAID) or high alcohol consumption.
This study was performed with the approval of the ethics committee of Tongji Medical College, Huazhong University of Science and Technology in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment in the study.
Endoscopic evaluation
According to standard procedure, the bowel preparation was performed with polyethylene glycol electrolyte solution before endoscopic work‐up. Patients received EGD (GIF‐Q260; Olympus, Tokyo, Japan) and/or ileocolonoscopy (CF‐Q260; Olympus). Standard endoscopies were performed, and the type of disease and activity of intestinal inflammation were evaluated by senior gastroenterologists who were blinded to fecal marker values at the time of investigation. Biopsies were taken if necessary, as decided by the performers.
Determination of the final diagnosis
For the purpose of this analysis, the final diagnosis included the following items: normal findings, gastric polyps, esophagitis/gastritis/duodenitis, peptic ulcer, gastric cancer, colorectal polyps, UC, Crohn’s disease (CD) and colorectal cancer (CRC), etc. For those who had undergone EGD first but without significant findings, colonoscopy was performed next, and vice versa. Only those subjects with no positive findings in either EGD or colonoscopy were considered normal. Two gastroenterologists blinded to the study hypothesis and fecal marker values independently established the final diagnosis based on all available patient‐related medical records, including clinical history, laboratory results, endoscopy reports and histological findings. When there was more than one diagnosis, the severity of mucosal inflammation determined the final diagnosis.
Fecal sample extraction
Fecal samples were weighed and reconstituted with extraction buffer [calprotectin enzyme‐linked immunosorbent assay (ELISA) kit] to obtain a final concentration at 400 mg/ml. The solution was then homogenized three times with an electrical homogenizer (Tissue Lyser‐24; Shanghai Jingxin Industrial Development Corporation, Shanghai, China) to obtain a homogeneous fecal suspension. After centrifugation at 14000 g for 15 min, the supernatants (fecal extracts) were collected and stored at –80℃ until quantitative measurement of fecal BAFF and calprotectin.
Distribution of BAFF in feces
In order to measure whether BAFF was evenly distributed throughout the feces, we randomly took three 500‐mg aliquots (spot sample) from the feces (n = 20), and the remaining fecal sample (2000 mg) was diluted in extraction buffer and then homogenized with tube homogenizers. The homogenized sample and spot samples were extracted as described, and BAFF levels in feces were measured by ELISA.
The BAFF levels in the three spot samples were similar to those in the corresponding blended feces, with no significant difference between unblended and blended samples. The correlations between one, two, and three spot samples and the corresponding blended sample were calculated, respectively, with Pearson’s correlation coefficient varying between 0·86 and 0·95. A scatterplot showing the correlation of BAFF in randomly collected and blended samples is presented in Fig. 1.
Figure 1.
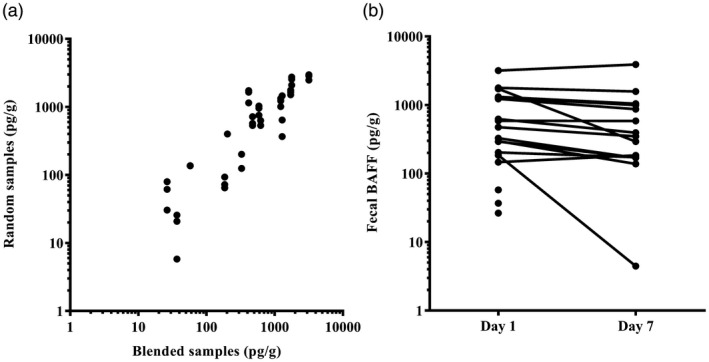
(a) Correlation between fecal B cell activating factor (BAFF) in three random spot samples and a corresponding homogenized sample (n = 20). Pearson’s correlation varied between 0·86 and 0·95 (P < 0·0001). (b) Fecal BAFF concentrations before and after 7 days of storage at 20°C; Mann–Whitney U‐test for pairwise comparisons (P = 0·2604).
Stability of BAFF in feces
In order to measure whether BAFF was stable in feces, the stool samples (n = 20) were divided into two aliquots of 1000 mg. One aliquot was immediately extracted and detected the level of fecal BAFF by ELISA, and the other was stored at 20°C for 7 days before extraction. There were no significant differences (P > 0·05) in BAFF levels before and after 7 days (Fig. 1).
Measurement of fecal BAFF and calprotectin
Commercially available ELISA kits were used. Fecal BAFF was quantitatively determined by the Quantikine human BAFF/BLyS/TNFSF13B immunoassay (R&D Systems, Inc., Minneapolis, MN, USA). The quantitative range was 62·5–4000 pg/ml, with the intra‐ and interassay coefficient variability ranging from 3·4–6·5% and 10·0–11·6%, respectively. The quantitative measurement of fecal calprotectin was performed using the Bühlmann fecal calprotectin TM ELISA kit (Bühlmann Laboratories AG, Schönenbuch, Switzerland). The quantitative range was 10–600 mg/g (lower‐range ELISA procedure) and 30–1800 µg/g (extended‐range ELISA procedure). Based on the previous experiment, the lower‐range procedure with a working range of 10–600 µg/g was adopted after diluting the sample according to the manufacturer’s instructions. The intra‐ and interassay coefficient variabilities were 4·7% and <1·5%, respectively.
Fecal BAFF and calprotectin were measured at a concentration of 400 mg/ml in fecal extraction. If the measured levels of BAFF or calprotectin exceed the upper limit, samples were further diluted and measured again to obtain a quantitative value. All measurements were carried out in accordance with the manufacturer’s test instructions. ELISA plates were read on a microtiter plate reader (Tecan Infinite F50) with an absorbance of 450 nm. The technician carrying out the analyses was unaware of the study hypothesis and the patient’s diagnosis.
Statistical analysis
Data were recorded by Microsoft Office Excel 2010. Statistical analysis was performed with the statistical software package spss version 13.0. Results are presented as mean with standard deviation (s.d.) for parametric numerical data and as median with interquartile range (IQR) for non‐parametric numerical data. Categorical data are summarized as the percentage of the total number of groups. Non‐parametric numeric data in two independent groups were compared using the Mann–Whitney U‐test and categorical data in two independent groups were compared using the c2 test. The correlation between fecal BAFF and calprotectin was analyzed by Pearson’s correlation coefficient (r). Receiver operating characteristics (ROC) curve was performed to determine the optimal cut‐off values and the test characteristics of fecal biomarkers to determine a clinically significant finding in the gastrointestinal tract. The test characteristics were characterized by sensitivity, specificity, positive and negative predictive value (PPV, NPV). A P‐value of <0·05 was considered statistically significant.
Results
Patient characteristics
A total of 230 patients with gastrointestinal symptoms were prospectively enrolled. Among them, 38 patients (16%) were excluded (mainly due to gastrointestinal surgery, incomplete endoscopy and poor adherence with fecal sampling). Finally, 192 patients (122 males/70 females; median age = 48 years; age range = 14–84 years) were included in the analysis. Baseline characteristics of enrolled patients are summarized in Table 1.
Table 1.
Patient characteristics
| Diagnosis | Numbera | Gender (male)a | Age (years)b |
|---|---|---|---|
| Normal controls | 44 (23%) | 27 (61%) | 39 (32–51) |
| Gastric polyps | 13 (7%) | 6 (46%) | 50 (48–60) |
| Esophagitis/gastritis/duodenitis | 47 (24%) | 27 (57%) | 48 (43–53) |
| Peptic ulcer | 11 (6%) | 7 (64%) | 46 (36–53) |
| Gastric cancer | 21 (11%) | 14 (67%) | 59 (50–63) |
| Colorectal polyps | 11 (6%) | 9 (82%) | 49 (44–61) |
| Ulcerative colitis | 12 (6%) | 11 (92%) | 40 (32–52) |
| Crohn’s disease | 16 (8%) | 12 (75%) | 35 (24–45) |
| Colorectal cancer | 17 (9%) | 9 (53%) | 59 (51–64) |
Count (%);
median (interquartile range).
Fecal BAFF and calprotectin in patients with abdominal discomfort
The median values of fecal BAFF and calprotectin are demonstrated in Table 2, and individual values are plotted on scatterplots to visually observe the differentiation (Fig. 2). Comparisons between the diagnostic group and healthy controls using Mann–Whitney tests are also shown (Table 2). In the upper gastrointestinal diseases, fecal BAFF and calprotectin in patients with gastric cancer were apparently higher than that in the normal group (P < 0·0001), while the values of fecal BAFF and calprotectin in patients with gastric polyps, esophagitis/gastritis/duodenitis and peptic ulcer were in the range of the normal group (P > 0·05). In the lower gastrointestinal diseases, fecal BAFF and calprotectin in UC and CD groups were significantly higher than those of controls (P < 0·0001). Statistically significant differences in fecal BAFF and calprotectin concentrations were also found in patients with colorectal cancer. There was no significant difference between colon polyps and controls (P > 0·05).
Table 2.
Fecal BAFF and calprotectin values in common gastrointestinal diseases
| Diagnosis | Number | BAFFa (pg/g) | P‐value | Calprotectina (μg/g) | P‐value |
|---|---|---|---|---|---|
| Healthy controls | 44 | 61 (33–68) | – | 17 (14–37) | – |
| Gastric polyps | 13 | 36 (8–89) | 0·5617 | 37 (5–104) | 0·4810 |
| Esophagitis/gastritis/duodenitis | 47 | 53 (0–123) | 0·6103 | 27 (6–70) | 0·7716 |
| Peptic ulcer | 11 | 56 (0–369) | 0·8580 | 36 (0–136) | 0·2302 |
| Gastric cancer | 21 | 267 (79–503) | <0·0001 | 104 (31–145) | <0·0001 |
| Colorectal polyps | 11 | 71 (35–82) | 0·4550 | 5 (0–39) | 0·2869 |
| Ulcerative colitis | 12 | 1549 (758–3196) | <0·0001 | 325 (196–1281) | <0·0001 |
| Crohn’s disease | 16 | 735 (263–1027) | <0·0001 | 181 (114–285) | <0·0001 |
| Colorectal cancer | 17 | 533 (308–1620) | <0·0001 | 200 (59–438) | <0·0001 |
Median (interquartile range). P‐value = difference between the levels of fecal markers in the diagnostic groups compared to that in controls.
Mann–Whitney U‐test for independent comparisons. BAFF = B cell activating factor.
Figure 2.
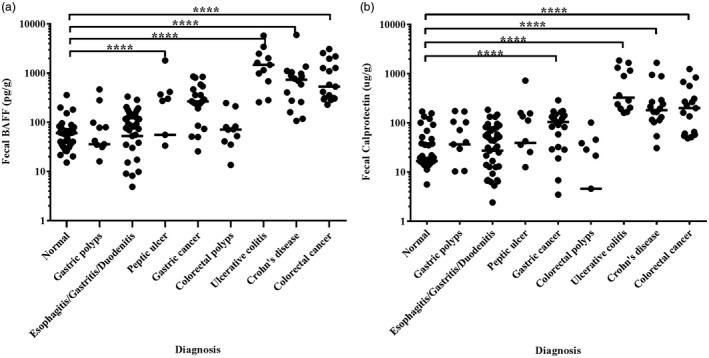
The values of fecal BAFF and calprotectin in each group were respectively plotted on the scatterplot. Solid lines indicate median levels. ***P < 0·0001.
Based on the above‐mentioned results, we further classified enrolled patients into the following four categories: normal, cancer (gastric cancer and colorectal cancer), IBD (UC and CD) and other diseases (gastric polyps, esophagitis/gastritis/duodenitis, peptic ulcer and colorectal polyps) groups. Fecal BAFF and calprotectin concentrations are significantly elevated in gastrointestinal neoplasms and IBD patients (Fig. 3). The median values are listed in Table 3.
Figure 3.
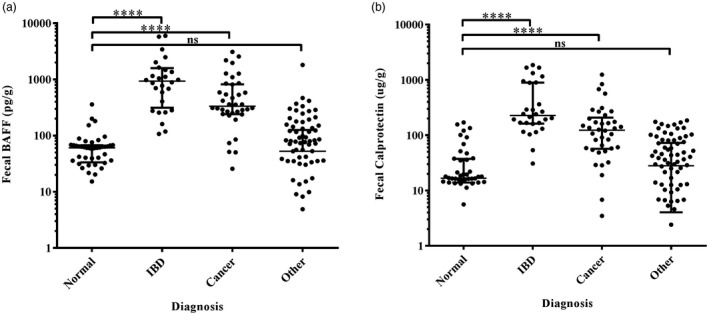
Fecal B cell activating factor (BAFF) and calprotectin in different diagnostic groups. IBD = inflammatory bowel diseases; ****P < 0·0001; n.s. = non‐significant.
Table 3.
Fecal BAFF and calprotectin values in different diagnostic groups
| Diagnosis | Number | BAFFa(pg/g) | P‐value | Calprotectina (μg/g) | P‐value |
|---|---|---|---|---|---|
| Normal | 44 | – | – | 17 (14–37) | – |
| IBD | 28 | 932 (313–1587) | <0·0001 | 227 (162–895) | <0·0001 |
| Cancer | 38 | 333 (240–819) | <0·0001 | 123 (57–207) | <0·0001 |
| Other | 82 | 53 (0–126) | 0·7974 | 28 (4–72) | 0·7522 |
Median (interquartile range);
P‐value, difference between the levels of fecal markers in the diagnostic groups compared to that in controls.
Mann–Whitney U‐test for independent comparisons.
IBD = inflammatory bowel diseases; BAFF = B cell activating factor.
Diagnostic value of fecal BAFF and calprotectin to select cancer and IBD in patients with abdominal discomfort
Using ROC analysis, we calculated an optimized cut‐off value for fecal BAFF and calprotectin to distinguish patients with IBD or gastroenteric tumor (Fig. 4). For IBD, fecal BAFF at 253·5 pg/g had a sensitivity of 89%, specificity 77%, PPV 40% and NPV 98%. Corresponding values for fecal calprotectin at 103·2 mg/g were 93, 77, 41 and 98%, respectively. For tumor, the performance of BAFF at a level of 186·7 pg/g to discriminate gastrointestinal cancer from other diseases showed a sensitivity of 84%, specificity 74%, PPV 44% and NPV 95%. The global cut‐off point of fecal calprotectin was 47·8 µg/g (sensitivity 84%, specificity 59%, PPV 34% and NPV 94%). For IBD and tumor, at a level of 219·5 pg/g the sensitivity of the BAFF test was 85%, while the specificity was 91%, with NPV and PPV of 84 and 92%, respectively. Fecal calprotectin at a level of 50·8 mg/g was observed at a sensitivity of 86% and specificity of 75% for IBD and tumor. We also investigated the diagnostic performance of a composite score combining fecal BAFF and calprotectin, which is summarized in Table 4. Furthermore, a significant correlation was detected between fecal calprotectin and BAFF concentration, shown in Fig. 5 [r = 0·7292, 95% confidence interval (CI) = 0·6552–0·7893, P < 0·0001].
Figure 4.
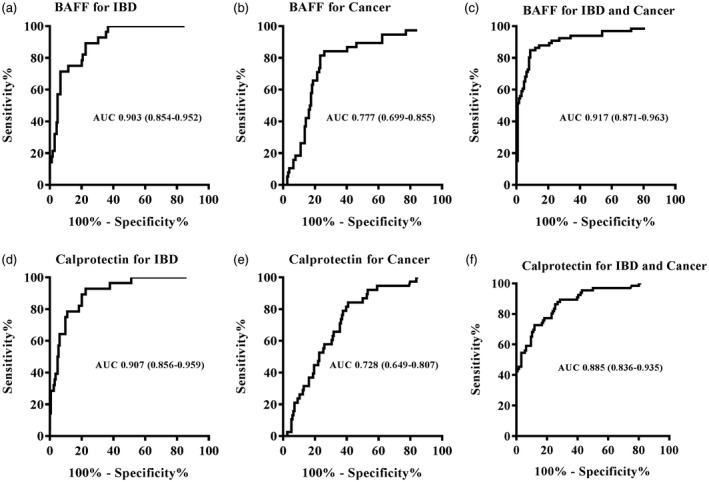
Receiver operator characteristic (ROC) curves for fecal B cell activating factor (BAFF) (a–c) and calprotectin (d,e) to discriminate inflammatory bowel disease (IBD) or/and cancer from other common gastrointestinal diseases. Data were described by area under the curve (AUC) with 95% confidence interval (CI) in parentheses.
Table 4.
Performance of fecal BAFF and calprotectin in gastrointestinal cancer and inflammatory bowel diseases
| BAFF | Cut‐off value (pg/g) | SEN (%) | SPE (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Cancer | 186·70 | 84 | 74 | 44 | 95 |
| IBD | 253·50 | 89 | 77 | 40 | 98 |
| Cancer and IBD | 219·50 | 85 | 91 | 84 | 92 |
| Calprotectin | Cut‐off value (μg/g) | SEN (%) | SPE (%) | PPV (%) | NPV (%) |
| Cancer | 47·80 | 84 | 59 | 34 | 94 |
| IBD | 103·20 | 93 | 77 | 41 | 98 |
| Cancer and IBD | 52·84 | 86 | 75 | 64 | 91 |
| BAFF and calprotectin | Cut‐off value | SEN (%) | SPE (%) | PPV (%) | NPV (%) |
| Cancer | 186·70 + 47·80 | 92 | 47 | 30 | 96 |
| IBD | 253·50 + 103·20 | 96 | 65 | 32 | 99 |
| Cancer and IBD | 219·50 + 52·84 | 92 | 61 | 55 | 94 |
The cut‐off value determined by receiver operator characteristic (ROC) curves.
SEN = sensitivity; SPE = specificity; PPV = positive predictive value; NPV = negative predictive value; BAFF = B cell activating factor; IBD = inflammatory bowel disease.
Figure 5.
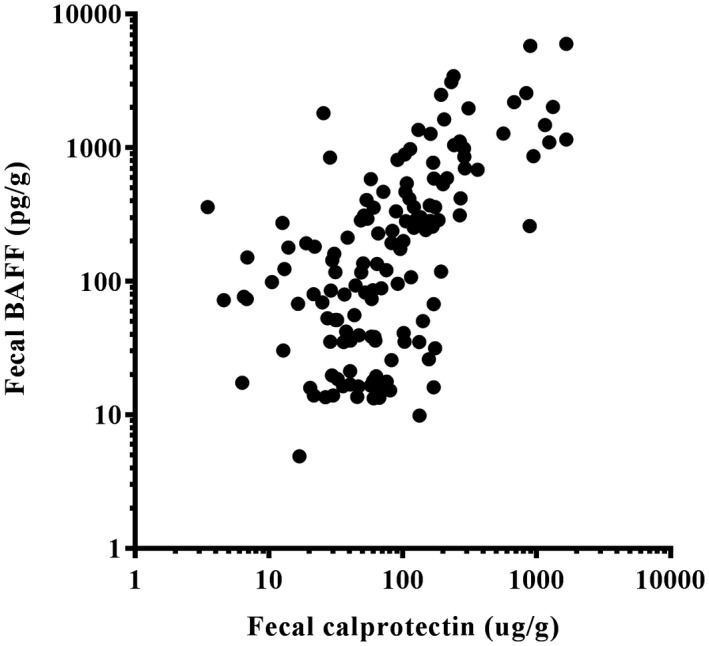
Correlation between fecal B cell activating factor (BAFF) and fecal calprotectin assay. Pearson’s rank correlation coefficient r = 0·7292 [95% confidence interval (CI) = 0·6552–0·7893], P < 0·0001.
Discussion
BAFF has recently been identified as a novel stool‐based marker of IBD. We have reported for the first time, to our knowledge, that the levels of BAFF in serum, feces and colon tissues of IBD patients are higher than those of IBS patients and healthy volunteers, and serum BAFF levels were closely correlated to clinical activity, CRP and various proinflammatory cytokines in serum, such as TNF‐α and IL‐1β 21. In addition, fecal BAFF concentrations in patients with IBD were higher compared to those in patients with IBS and healthy controls, which allowed correctly distinguishing patients with a high specificity and sensitivity of 100 and 84% at the optimal cut‐off value of 227·3 pg/ml, while the PPV and NPV were 100 and 64%, respectively. Moreover, there is a highly significant correlation between fecal BAFF with endoscopic inflammation severity, which is closer than that between calprotectin and mucosal inflammation. Therefore, fecal BAFF can be used as a promising stool marker for identifying IBD and evaluating mucosal inflammation 22. However, whether fecal BAFF can be put into use in detecting other organic gastrointestinal diseases, such as gastrointestinal tumor, remains to be investigated. Thus, this prospective study was carried out to examine the usefulness of fecal BAFF as a screening test for patients with abdominal discomfort compared with fecal calprotectin. We measured fecal BAFF and calprotectin as a comparison in a cohort of consecutive patients undergoing endoscopy for abdominal discomfort.
The main characteristics of fecal biomarkers include the stability of fecal samples and the existence of a sensitive and reliable detection assay 24. Similarly, BAFF was found to be temperature‐stable for 7 days and equally distributed in the feces in this study. Therefore, stool specimens for BAFF measurement can be mailed to the laboratory without specific storage conditions, and only a small number of samples were needed to accurately measure BAFF in feces. With these valuable characteristics, fecal BAFF is considered to have great potential as a non‐invasive disease marker.
In this study, the current results confirm our previous findings that fecal BAFF concentrations were significantly higher in IBD patients. Furthermore, fecal BAFF proved to be a valuable non‐invasive marker that was able to accurately distinguish patients with IBD from patients without in this unselected group of patients with gastrointestinal symptoms, giving a sensitivity of 89%, specificity 77%, PPV of 40% and NPV of 98%. Previous studies have demonstrated that calprotectin also shows excellent performance in discriminating between such groups, with sensitivity values ranging from 70 to 90% and specificities of a similar order in a referred population 25. Our results are very close to previously reported values. Von Roon et al. performed a meta‐analysis which showed that the diagnostic accuracy of fecal calprotectin for IBD at a cut‐off value of 100 µg/g appeared to be better than at 50 µg/g 26. As can be seen from the results, the ability of the fecal BAFF test for diagnosing IBD is similar to that of fecal calprotectin, so it may be recommended as a useful screening tool for identifying suspected IBD patients who are most likely to have endoscopies, which will essentially reduce the number of invasive examinations and unnecessary costs.
The survival rate of gastrointestinal cancer is closely correlated with the stage of diagnosis, so early detection could reduce the mortality rate 27. Colonoscopy remains the gold standard for detecting malignant gastrointestinal tumors, but it cannot represent a reliable primary screening tool because of its shortcomings. By contrast, fecal examination has been recommended as a non‐invasive and relatively inexpensive screening approach 28. Fecal occult blood (FOB), the most widely used screening method, reported a 15–30% reduction of mortality, with relatively high sensitivity but somewhat low NPV. Calprotectin was also higher in patients with gastrointestinal tumors. However, a study which specifically detected calprotectin in 2321 individuals showed a sensitivity of 76% for any neoplasia and 27% for advanced neoplasia, which indicated that fecal calprotectin cannot be recommended for colorectal screening because of its undesirable performance 29. Similarly, a meta‐analysis concluded that the overall sensitivity and specificity of fecal calprotectin in the diagnosis of colorectal cancer were 36 and 71%, and that it was not recommended as a screening test for colorectal malignancies in the general population 30. Among 39 patients with known esophageal gastric cancer and 191 control subjects, fecal calprotectin had a sensitivity of 76.9% and a specificity of 88.0% in the diagnosis of esophageal gastric cancer, using the manufacturer’s cut‐off value <50 mg/g 31. Our previous studies have shown that fecal BAFF was elevated in IBD patients, but its role in gastrointestinal cancer is still unknown. This is the first study to demonstrate that fecal BAFF was higher in patients with gastrointestinal tumor, including gastric and colorectal cancer. The data presented here have shown that fecal BAFF has a sensitivity of 84% and a specificity of 74% in the diagnosis of gastrointestinal cancer using a cut‐off of 186·7 pg/g. Fecal immunochemical tests (FITs) for hemoglobin (Hb) are widely recommended and used for CRC screening in many countries 32, 33, 34, 35 , as they have been shown to yield substantially better diagnostic performance in direct comparisons with guaiac‐based fecal occult blood tests (gFOBTs) 36, 37, 38. Previous studies have demonstrated large differences in the diagnostic performance of different quantitative FITs, with some of them showing very high sensitivity at very low specificity, and vice versa, which probably mainly reflect differences in the thresholds according to different manufacturers 39. The sensitivities of FITs for detecting CRC range from 25 to 100%, and also as specificities 40. Brenner and Tau 38 performed a head‐to‐head study and showed that the sensitivities, specificities, PPV and NPV of three FITs for CRC ranged from 53.3–73.3, 95.4–95.5, 7.3–10.0 and 99.7–99.8%, respectively. It is clear that fecal BAFF has higher PPV but lower specificity than FIT in detecting CRC.
Our study further demonstrated that fecal BAFF and calprotectin could represent useful non‐invasive biomarkers of IBD and tumor with sensitivities of 85 and 86%, respectively, and specificities of 91 and 75%. The PPVs of BAFF and calprotectin for IBD and tumor are 84 and 64%, respectively. BAFF and calprotectin have highly similar NPVs of 92 and 91%, respectively, in differentiating IBD and tumor from other diseases. As a diagnostic test for IBD and gastrointestinal tumor, we further wondered whether a composite score combining fecal BAFF and calprotectin would have better performance than the two separately. As shown in Table 4, an improved sensitivity with a reduction of specificity and PPV were observed when fecal BAFF and calprotectin were combined, while the NPV was almost the same. At present, invasive endoscopy is strongly recommended for patients with disturbing gastrointestinal symptoms, especially for those aged above 45 years, to rule out malignant tumor, but the screening criterion is too radical and causes many dispensable endoscopies. We have confirmed, in our study, that fecal BAFF gave high sensitivity and specificity for IBD and gastrointestinal cancer, and the sensitivity was even higher when combining fecal BAFF and calprotectin together. Accordingly, we are in favor of undertaking fecal BAFF examinations or combining with calprotectin before carrying out endoscopies in those patients with abdominal discomfort, which can possibly provide valuable diagnostic assistance in these unselected patient populations and enable physicians to identify those with high risks.
Munari et al. confirmed that BAFF was abundant in the mucosa from Helicobacter + patients with chronic gastritis, and it was accumulated in macrophages in‐vivo and was produced by monocyte‐derived macrophages in vitro after H. pylori stimulation 41, but in our study fecal BAFF was not elevated as a whole in patients with chronic gastritis. It is necessary to further increase the sample size and analyze the concentration of BAFF separately in feces according to H. pylori infection.
Our research has some limitations. First, the study only included a limited number of gastrointestinal diseases, but some diseases that possibly caused abdominal discomfort were not analyzed. Secondly, some small bowel diseases possibly cause increases in fecal BAFF and calprotectin concentrations, which may be an explanation of why elevated BAFF and calprotectin has been found in some patients with negative findings in EGD and colonoscopy. Thirdly, a relatively small number of cases were enrolled into this study, and it is necessary to expand the sample size for further research and more solid case–control studies need to be investigated in future.
Conclusion
In conclusion, our results support the perspective that fecal BAFF is a novel promising non‐invasive biomarker that can discriminate patients with IBD and malignancy from other gastrointestinal disease as effectively as fecal calprotectin. We confirmed our prior study, showing the great ability of fecal BAFF to recognize IBD. In addition, we expanded the role of fecal BAFF to gastrointestinal cancer as an auxiliary diagnostic tool, including gastric carcinoma and colorectal cancer. From our viewpoint, fecal BAFF tests are worth carrying out prior to endoscopic investigation, because this biomarker‐guided strategy might help physicians to establish diagnosis and make more appropriate decisions on endoscopy. Certainly, additional studies are required to further define the role of fecal BAFF as a non‐invasive inflammatory marker.
Disclosures
The authors declare no conflicts of interest relevant to the manuscript.
Author contributions
Planning and conducting the study: Y. F., C. X., R. Q.; collecting data and statistical analysis: L. W., C. C., C. X.; drafting and editing the manuscript: C. X., R. Q.; and critical revision of manuscript for intellectual content: Y. F., W. Y.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant number 81570501, 81770554, 81772607, 81472311).
References
- 1. Kay L, Jorgensen T. Abdominal symptom associations in a longitudinal study. Int J Epidemiol 1993; 22:1093–100. [DOI] [PubMed] [Google Scholar]
- 2. Kay L, Jorgensen T, Schultz‐Larsen K. Abdominal pain in a 70‐year‐old Danish population. An epidemiological study of the prevalence and importance of abdominal pain. J Clin Epidemiol 1992; 45:1377–82. [DOI] [PubMed] [Google Scholar]
- 3. Desai D, Faubion WA, Sandborn WJ. Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther 2007; 25:247–55. [DOI] [PubMed] [Google Scholar]
- 4. Vermeire S, Assche GV, Rutgeerts P. Role of biomarkers in the diagnosis of inflammatory bowel disease. Expert Opin Med Diagn 2007; 1:481–8. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen OH, Vainer B, Madsen SM et al Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol 2000; 95:359–67. [DOI] [PubMed] [Google Scholar]
- 6. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 2006; 55:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gisbert JP, Gomollon F, Mate J et al The role of anti‐neutrophil cytoplasmic antibodies (ANCA) and anti‐Saccharomyces cerevisiae antibodies (ASCA) in inflammatory bowel disease. Gastroenterol Hepatol 2003; 26:312–24. [DOI] [PubMed] [Google Scholar]
- 8. The Danish Dyspepsia Study Group . Value of the unaided clinical diagnosis in dyspeptic patients in primary care. Am J Gastroenterol 2001; 96:1417–21. [DOI] [PubMed] [Google Scholar]
- 9. Jellema P, van der Windt DA, Schellevis FG et al Systematic review: accuracy of symptom‐based criteria for diagnosis of irritable bowel syndrome in primary care. Aliment Pharmacol Ther 2009; 30:695–706. [DOI] [PubMed] [Google Scholar]
- 10. Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009; 9:491–502. [DOI] [PubMed] [Google Scholar]
- 11. Striz I, Brabcova E, Kolesar L et al Cytokine networking of innate immunity cells: a potential target of therapy. Clin Sci (Lond) 2014; 126:593–612. [DOI] [PubMed] [Google Scholar]
- 12. Mackay F, Figgett WA, Saulep D et al B cell stage and context‐dependent requirements for survival signals from BAFF and the B cell receptor. Immunol Rev 2010; 237:205–25. [DOI] [PubMed] [Google Scholar]
- 13. Thangarajh M, Gomes A, Masterman T et al Expression of B cell‐activating factor of the TNF family (BAFF) and its receptors in multiple sclerosis. J Neuroimmunol 2004; 152:183–90. [DOI] [PubMed] [Google Scholar]
- 14. Vincent FB, Morand EF, Schneider P et al The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 2014; 10:365–73. [DOI] [PubMed] [Google Scholar]
- 15. Wei F, Chang Y, Wei W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine 2015; 76:537–44. [DOI] [PubMed] [Google Scholar]
- 16. Cheema GS, Roschke V, Hilbert DM et al Elevated serum B lymphocyte stimulator levels in patients with systemic immune‐based rheumatic diseases. Arthritis Rheum 2001; 44:1313–9. [DOI] [PubMed] [Google Scholar]
- 17. Roschke V, Sosnovtseva S, Ward CD et al BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune‐based rheumatic diseases. J Immunol 2002; 169:4314–21. [DOI] [PubMed] [Google Scholar]
- 18. Krumbholz M, Derfuss T, Hohlfeld R et al B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol 2012; 8:613–23. [DOI] [PubMed] [Google Scholar]
- 19. Candon S, Gottenberg JE, Bengoufa D et al Quantitative assessment of antibodies to ribonucleoproteins in primary Sjogren syndrome: correlation with B cell biomarkers and disease activity. Ann Rheum Dis 2009; 68:1208–12. [DOI] [PubMed] [Google Scholar]
- 20. Jonsson MV, Szodoray P, Jellestad S et al Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjogren's syndrome. J Clin Immunol 2005; 25:189–201. [DOI] [PubMed] [Google Scholar]
- 21. Zhang P, Liu X, Guo A et al B cell‐activating factor as a new potential marker in inflammatory bowel disease. Dig Dis Sci 2016; 61:2608–18. [DOI] [PubMed] [Google Scholar]
- 22. Fu Y, Wang L, Xie C et al Comparison of non‐invasive biomarkers faecal BAFF, calprotectin and FOBT in discriminating IBS from IBD and evaluation of intestinal inflammation. Sci Rep 2017; 7:2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manz M, Burri E, Rothen C et al Value of fecal calprotectin in the evaluation of patients with abdominal discomfort: an observational study. BMC Gastroenterol 2012; 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pardi DS, Sandborn WJ. Predicting relapse in patients with inflammatory bowel disease: what is the role of biomarkers? Gut 2005; 54:321–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta‐analysis. BMJ 2010; 341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Roon AC, Karamountzos L, Purkayastha S et al Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol 2007; 102:803–13. [DOI] [PubMed] [Google Scholar]
- 27. Pezzilli R, Barassi A, Morselli LA et al Fecal calprotectin levels in patients with colonic polyposis. Dig Dis Sci 2008; 53:47–51. [DOI] [PubMed] [Google Scholar]
- 28. Limburg PJ, Devens ME, Harrington JJ et al Prospective evaluation of fecal calprotectin as a screening biomarker for colorectal neoplasia. Am J Gastroenterol 2003; 98:2299–305. [DOI] [PubMed] [Google Scholar]
- 29. Hoff G, Grotmol T, Thiis‐Evensen E et al Testing for faecal calprotectin (PhiCal) in the Norwegian Colorectal Cancer Prevention trial on flexible sigmoidoscopy screening: comparison with an immunochemical test for occult blood (FlexSure OBT). Gut 2004; 53:1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Roon AC, Karamountzos L, Purkayastha S et al Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol 2007; 102:803–13. [DOI] [PubMed] [Google Scholar]
- 31. Vincent Z, Hornby S, Ball S et al Faecal calprotectin as a marker for oesophago‐gastric cancer. Ann Clin Biochem 2015; 52:660–4. [DOI] [PubMed] [Google Scholar]
- 32. Halloran SP, Launoy G, Zappa M. International Agency for Research on Cancer European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—faecal occult blood testing. Endoscopy 2012; 44:SE65–SE87. [DOI] [PubMed] [Google Scholar]
- 33. US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016; 15:2564–75. [Google Scholar]
- 34. Wolf AMD, Fontham ETH, Church TR et al Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018; 68:250–81. [DOI] [PubMed] [Google Scholar]
- 35. Benard F, Barkun AN, Martel M, von Renteln D. Systematic review of colorectal cancer screening guidelines for average‐risk adults: summarizing the current global recommendations. World J. Gastroenterol 2018; 24:124–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu MM, Xu XT, Nie F, Tong JL, Xiao SD, Ran ZH. Comparison of immunochemical and guaiac‐based fecal occult blood test in screening and surveillance for advanced colorectal neoplasms: a meta‐analysis. J Dig Dis 2010; 11:148–60. [DOI] [PubMed] [Google Scholar]
- 37. Park DI, Ryu S, Kim YH et al Comparison of guaiac‐based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol 2010; 105:2017–25. [DOI] [PubMed] [Google Scholar]
- 38. Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head‐to‐head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013; 49:3049–54. [DOI] [PubMed] [Google Scholar]
- 39. Gies A, Cuk K, Schrotz‐King P, Brenner H. Combination of different fecal immunochemical tests in colorectal cancer screening: any gain in diagnostic performance? Cancers (Basel) 2019; 11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gies A, Bhardwaj M, Stock C, Schrotz‐King P, Brenner H. Quantitative fecal immunochemical tests for colorectal cancer screening. Int J Cancer 2018; 143:234–44. [DOI] [PubMed] [Google Scholar]
- 41. Munari F, Fassan M, Capitani N et al Cytokine BAFF released by Helicobacter pylori‐infected macrophages triggers the Th17 response in human chronic gastritis. J Immunol 2014; 193:5584–94. [DOI] [PubMed] [Google Scholar]


