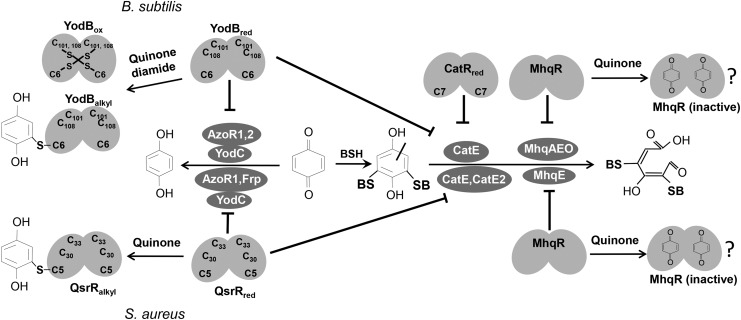
FIG. 10.
The roles of the quinone-sensing MhqR, CatR, and YodB/QsrR regulons in B. subtilis and S. aureus. Exposure of B. subtilis and S. aureus to quinones induces the quinone detoxification regulons controlled by the homologous MarR-type repressors MhqR, YodB/QsrR, and CatR. The redox-sensing MarR/DUF24-family repressors YodB and CatR of B. subtilis are inactivated by intersubunit disulfide formation in vivo that involves the conserved Cys6 or Cys7 (1, 2, 12, 13, 69). The YodB and QsrR repressor mutant proteins with single Cys6 and Cys5 sense quinones also by thiol-S-alkylation in vitro (33, 41). The MhqR repressors might be inactivated by direct binding of quinones to a specific pocket. The MhqR and YodB/QsrR regulon include homologous quinone reductases, nitroreductases, and dioxygenases for quinone and diamide detoxification. The thiol-dependent dioxygenases MhqA, MhqE, CatE, and MhqO of B. subtilis and their respective homologs CatE, MhqE, and CatE2 of S. aureus (Supplementary Fig. S3B) are involved in ring cleavage of quinone-S-adducts. The quinone reductases AzoR1 and AzoR2 of B. subtilis and AzoR1 and Frp of S. aureus and the nitroreductases YodC and MhqN of B. subtilis and YodC of S. aureus catalyze the reduction of quinones to redox stable hydroquinones.