Abstract
This cross-sectional study aimed to assess and compare quality of life in patients with advanced oral cavity tumors after mandibular resection in 3 groups (no reconstruction, reconstruction with plate, and reconstruction with flap) at the Cancer Institute, affiliated to Tehran University of Medical Sciences. Quality of life was measured using the European Organization for Research and Treatment of Cancer core quality of life questionnaire and European Organization for Research and Treatment of Cancer head and neck cancer-specific quality of life questionnaire-35 items. The comparison was tested using Kurskal–Wallis analysis. All 120 patients were entered into the study. The mean age of patients was 48.5 (standard deviation = 18.1) years. Patients presented with advanced stage of the disease and underwent mandibular resection with no reconstruction (n = 40), reconstruction with plate (n = 41), and reconstruction with flap (n = 39). The findings showed that in general, there were no statistically significant differences in quality of life among 3 groups except for speech problem (P = .4), dry mouth (P = .03), and feeling ill (P = .04). Although there were no significant differences in quality of life among patients in 3 groups, overall patients who received reconstruction with flap reported better functioning and fewer symptoms. Those who did not receive any reconstruction reported the worse conditions.
Keywords: cancer of oral cavity, flap reconstruction, head and neck cancer, mandibular resection, plate reconstruction, quality of life
1. Introduction
Treatment and surgery for head and neck cancers often causes anatomical changes that can lead to severe dysfunction in oral cavity such as difficulties in speech, chewing, and swallowing.[1] In addition, such treatments might interfere with patients’ appearance, pain, and suffering that all could influence quality of life in these patients.[2] These undesirable conditions are mostly occurring due to postoperative radiotherapy, which is often needed.[3] Until recently, neither restoration nor reconstruction, or the usual prosthetic techniques were able to resolve these problems successfully. Therefore, choosing an appropriate reconstruction technique seems to be an important parameter when treating these patients.[4]
However, regardless of different treatment regimens and reconstruction procedures, improving health-related quality of life remains essential for these patients. A study of patients with head and neck cancer showed that even greater than treatment modality, baseline quality of life and comorbidity influenced posttreatment quality of life.[5] Also, a long-term multicenter study of quality of life and psychosocial outcomes after oropharyngeal cancer surgery and radial forearm free-flap reconstruction reported that psychosocial distress was the main determinant of long-term quality of life and suggested that the multidisciplinary management of these patients is of prime importance.[6] Thus, this study aimed to have a more focused and detailed approach in evaluating quality of life in different patient groups to add to the existing knowledge on the topic.
2. Materials and methods
2.1. Design and patients
This was a cross-sectional study of quality of life in patients with oral cancer referred to Tehran Cancer Institute, affiliated to Tehran University of Medical Science during year 2017 and 2018 in Iran. All patients who received oral cancer surgery at the study period were identified and entered into the study if they survived 12 months following surgery. In all patients, the tumor was spread to the mandible bone, so that the treatment plan involved a resection of the mandible and the loss of bone continuity. Based on patients’ conditions including age, stage of the disease, postsurgical treatment, and clinical decision making, 3 groups of patients were identified: patients who underwent reconstruction with plate, patients who underwent reconstruction with flap, and those who did not receive any reconstruction. As indicated all patients completed a demographic and quality of life questionnaire at the end of 12 months follow-up. Clinical information was extracted from case records.
2.2. Quality of life questionnaires
Quality of life was measured using the following questionnaires.
2.2.1. The EORTC QLQ-C30
European Organization for Research and Treatment of Cancer core quality of life questionnaire (EORTC QLQ-C30) is a core questionnaire for measuring quality of life in patients with cancer. It consists of 30 items tapping into 6 functioning and a number of symptoms subscales. Scores for each subscale range from 0 to 100 where for the functioning subscales, the higher scores indicate better conditions and for the symptoms it is vice versa.[7] The psychometric properties of the Iranian version of the questionnaire are well documented.[8]
2.2.2. The EORTC QLQ-H&N35
European Organization for Research and Treatment of Cancer head and neck cancer-specific quality of life questionnaire-35 items (EORTC QLQ-H&N35) is the early version of a specific questionnaire for measuring quality of life in head and neck cancer. The questionnaire consists of 35 items measuring a number of symptoms including pain, swallowing, sense problems, speech problems, trouble with social eating, and trouble with social contact. Scores for each symptoms range from 0 to 100, where the higher scores indicate worse conditions.[9,10] The questionnaire is validated in Iran and its report is available elsewhere.[11]
2.3. Analysis
Descriptive statistics were used to explore the data. Given that the distribution of data was not normal, thus nonparametric test (Kurskal–Wallis test) was performed for comparing quality of life among 3 groups of patients. Since there were no significant differences among 3 groups with regard to age, gender, and tumor stage, we did not control for any confounding variables. Data were analyzed by SPSS software. The P < .05 was considered statistically significant.
2.4. Ethics
The ethics committee of Tehran University of Medical Sciences approved the study. All patients complete written informed consent before the study commence.
3. Results
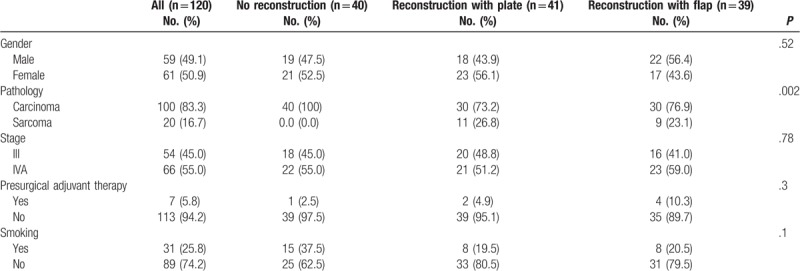
In all, there were 170 patients. Of these, 50 patients were died at 1-year follow-up. Thus, the remaining 120 patients were entered into the study (no reconstruction: 40, reconstruction with plate: 41, and reconstruction with flap: 39). The mean age of patients was 50.4 (standard deviation = 15.9) years, ranging from 22 to 85 with a median of 49 years. There were no significant differences among patients in age (P = .12), gender (P = .52) and stage of the disease (P = .78). Most patients presented with advanced stage (III and IVA) and all underwent mandibular resection. The characteristics of patients are presented in Table 1.
Table 1.
The characteristics of study samples.
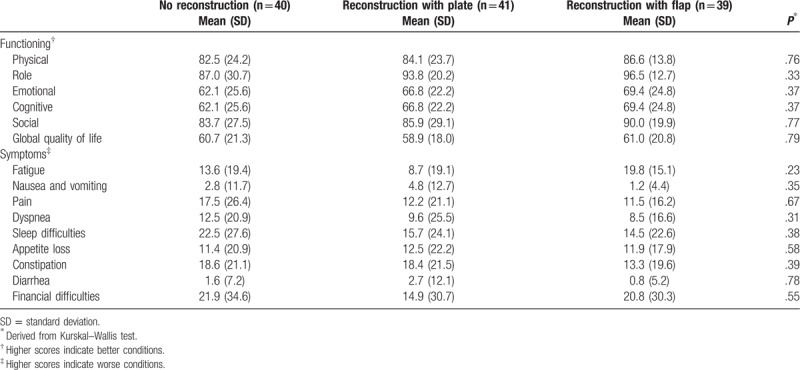
Quality of life as measured with the EORTC QLQ-C30 showed no significant differences among different patient groups. However, in general patients who received reconstruction with flap reported better quality of life compared to the other 2 groups. In addition, the findings showed that patients in 3 groups had relatively high scores on physical functioning, role functioning, and global quality of life, while they scored lower on emotional and cognitive functioning. Among symptoms fatigue and sleep difficulties were reported to be the most disturbing symptoms in 3 groups. The results are shown in Table 2.
Table 2.
Quality of life among study samples as measured by the European Organization for Research and Treatment of Cancer core quality of life questionnaire (EORTC QLQ-C30).
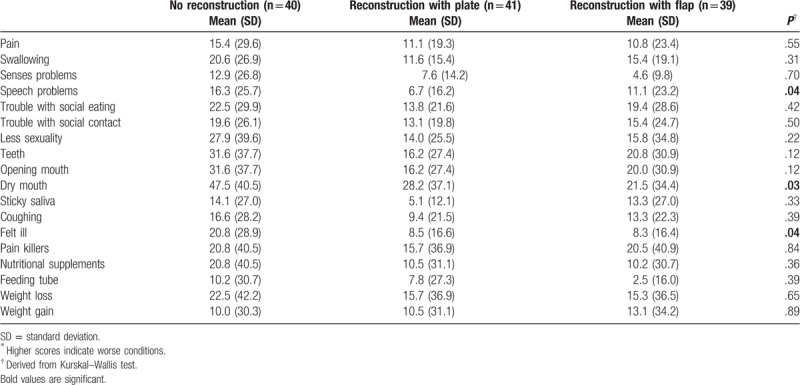
Finally, quality of life as assessed with the EORTC QLQ-H&N35 did not show any significant differences among the study samples except for speech problems (P = .04), dry mouth (P = .03), and feeling ill (P = .04). In general, patients who did not receive reconstruction scored higher on symptoms as measured by the EORTC QLQ-H&N35, indicating lower quality of life compared to patients who received reconstruction with plate or reconstruction with flap. The detailed results are presented in Table 3.
Table 3.
Quality of life among study samples as measured by the European Organization for Research and Treatment of Cancer head and neck cancer-specific quality of life questionnaire-35 items (EORTC QLQ-H&N35)∗.
4. Discussion
The findings from the current study did not show significant differences among patients who received different reconstructions for oral cancer. Unfortunately, there is a wide range of contradictions on reconstruction for cancers of oral cavity in the literature. Few studies reported on the type of complication with respect to the reconstruction parameters. Thus, it is very difficult to compare our surgical results, with other studies. For instance, Pandey et al in a randomized controlled trial of level IIb preserving neck dissection in clinically node-negative squamous carcinoma of the oral cavity using quality of life as the secondary outcome (measured by the Functional Assessment of Cancer Therapy head and neck cancer-specific questionnaire [FACT-HN] version 4 questionnaire) at the end of 1 year follow-up reported that no significant difference in quality of life was found in 2 study groups (IIb preserving superselective neck dissection vs conventional supraomohyoid neck dissection), although patients undergoing IIb preserving dissections had better general well-being, physical well-being, head and neck specific, and total FACT-HN scores at 6 months of follow-up.[12]
Landstrom in a study examined quality of life, survival, and treatment safety in 19 patients with primary cancers in the head and neck region who received electrochemotherapy (ECT). All patients except 1 had squamous cell carcinoma. Radiotherapy was also performed for all patients. The Performance Status Scale for Head & Neck Cancer Patients was used at baseline and 12 months after treatment to measure functional outcome. The results showed that no recurrence was occurred during the fallopian period but functional outcome in all parameters after 1 year of ECT significantly decreased and patients reported problems with taste and smell, talking, mouth opening, and dry mouth.[13]
Pierre et al examined functional health outcomes, postoperative quality of life after cancer surgery, and microvascular flap reconstruction in patients with oral or oropharyngeal cancer. Patients underwent surgery between 2000 and 2009 and were alive at least 1 year after treatment. Patients completed the Voice Handicap Index, the EORTC QLQ-C30, QLQ-H&N35, and the Dysphagia Outcome and Severity Scale. In this study, 64 patients were evaluated and the results showed that patients who underwent surgery for oral or oropharyngeal cancer with free-flap reconstruction had moderate but persistent functional and quality of life problems. The patients’ age, tumor stage, tumor location, and radiotherapy were found to be the main predictors of functional outcomes.[14] Similarly a study of 102 patients with head and neck cancer showed that reduced quality of life was associated with the clinical staging, patient's gender, and treatment approach. In fact, female patients who were diagnosed with advanced disease and underwent radiotherapy or chemotherapy were more likely to report lower rates of quality of life.[15]
A study examined depression and quality of life among survivors of head and neck cancers. A sample of patients with head and neck cancer (n = 209) with mean follow-up period of 38.7 months were evaluated by the EORTC QLQ-30, QLQ-H&N35, and the Hospital Anxiety Depression Scale. Significant pretreatment predictors of long-term depressive symptoms were cigarette smoking, alcohol use, T3 or T4 tumors, and more than 3 medications, while significant predictors of global quality of life were anemia, hypoalbuminemia, and T3 or T4 tumors.[16]
Functioning scores for our patients were relatively high. Such observation might be due to the fact that patients in current study were relatively young (mean age 50.4 years). However, although younger age might not critically influence functioning in these patients, younger age patients might suffer more from other consequences such as facial appearance. A study of 25 young patients reported that mandible reconstruction with fibula flap significantly influenced young patients’ quality of life.[17] However, a recent study reported that reconstructive microsurgery even can be proposed to older as well as younger patients because according to quality of life measures older patients can take advantage of this complex surgical technique.[18]
Three quality of life parameters were found significantly differed among the patients groups. These were speech problems, dry mouth, and felt ill. Looking at the data, it seems that the difference mainly was originated from difference between no reconstruction group with the 2 other groups; otherwise, there was no significant differences between the group who received reconstruction with plate or reconstruction with flap. A findings from a prospective study showed that in general microvascular reconstruction after mandibular osteoradionecrosis may improve health-related quality of life with an emphasis on pain reduction, improved scores for feeling ill, and sexual difficulties as measured by the EORTC QLQ-H&N35.[19]
The current research was a straightforward analysis of quality of life in 3 groups of patients following 12 months after surgery with a relatively small sample size. Thus, the findings from this study should be interpreted with caution since the baseline data were not available. In addition, we used the EORTC QLQ-H&N35. Although it is a valid measure, it is suggested that in studies investigating different treatment or targeted therapies, the QLQ-H&N43 (the newer version of the questionnaire) might be more suitable to detect differences between patient groups.[20] The Iranian version of the QLQ-H&N43 was not available at the study commence and still is not available.
5. Conclusion
There were no significant differences in quality of life scores among patients who underwent no reconstruction, reconstruction with plate, and reconstruction with plate except for speech problems, dry mouth, and feeling ill. Overall, the findings indicated that patients in “no reconstruction” group reported worse conditions compared to patients who underwent the plate or flap reconstructions.
Acknowledgment
The authors are grateful to all patients who participated in the study. They are also grateful to all academic staff in Tehran Cancer Institute for help and support.
Author contributions
Conceptualization: Mahammad M. Davudov, Iraj Harirchi, Ata Garajei, Mohammad Shirkhoda, Ali Montazeri.
Data curation: Mahammad M. Davudov, Ali Arabkheradmand, Habibollah Mahmudzadeh, Mohammad Shirkhoda, Maziar Motiee-Langroudi, Zoheir Mirzajani, Jayran Zebardast.
Formal analysis: Ali Montazeri.
Investigation: Mahammad M. Davudov, Ali Arabkheradmand, Mohammad Shirkhoda.
Methodology: Iraj Harirchi, Ata Garajei, Habibollah Mahmudzadeh, Maziar Motiee-Langroudi, Ali Montazeri.
Project administration: Iraj Harirchi.
Resources: Iraj Harirchi, Ali Arabkheradmand, Ata Garajei, Habibollah Mahmudzadeh, Mohammad Shirkhoda.
Software: Jayran Zebardast.
Supervision: Iraj Harirchi, Ali Montazeri.
Validation: Maziar Motiee-Langroudi, Jayran Zebardast.
Visualization: Zoheir Mirzajani, Jayran Zebardast, Ali Montazeri.
Writing – original draft: Mahammad M. Davudov, Zoheir Mirzajani.
Writing – review & editing: Ali Montazeri.
Ali Montazeri orcid: 0000-0002-5198-9539.
Footnotes
Abbreviations: ECT = electrochemotherapy, EORTC QLQ-C30 = European Organization for Research and Treatment of Cancer core quality of life questionnaire, EORTC QLQ-H&N35 = European Organization for Research and Treatment of Cancer head and neck cancer-specific quality of life questionnaire-35 items, EORTC QLQ-H&N43 = European Organization for Research and Treatment of Cancer head and neck cancer-specific quality of life questionnaire-43 items, FACT-HN = Functional Assessment of Cancer Therapy head and neck cancer-specific questionnaire.
How to cite this article: Davudov MM, Harirchi I, Arabkheradmand A, Garajei A, Mahmudzadeh H, Shirkhoda M, Motiee-Langroudi M, Mirzajani Z, Zebardast J, Montazeri A. Evaluation of quality of life in patients with oral cancer after mandibular resection. Medicine. 2019;98:41(e17431).
The authors report no conflicts of interest.
References
- [1].Denaro N, Merlano MC, Russi EG. Dysphagia in head and neck cancer patients: pretreatment evaluation, predictive factors, and assessment during radio-chemotherapy, recommendations. Clin Exp Otorhinolaryngol 2013;6:117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wong HM. Oral complications and management strategies for patients undergoing cancer therapy. Sci World J 2014;2014:581795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mosel DD, Bauer RL, Lynch DP, et al. Oral complications in the treatment of cancer patients. Oral Dis 2011;17:550–9. [DOI] [PubMed] [Google Scholar]
- [4].Kolokythas A. Long-term surgical complications in the oral cancer patient: a comprehensive review. Part I. J Oral Maxillofac Res 2010;1:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].El-Deiry MW, Futran ND, McDowell JA, et al. Influences and predictors of long-term quality of life in head and neck cancer survivors. Arch Otolaryngol Head Neck Surg 2009;135:380–4. [DOI] [PubMed] [Google Scholar]
- [6].Bozec A, Demez P, Gal J, et al. Long-term quality of life and psycho-social outcomes after oropharyngeal cancer surgery and radial forearm free-flap reconstruction: a GETTEC prospective multicentric study. Surg Oncol 2018;27:23–30. [DOI] [PubMed] [Google Scholar]
- [7].Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J Natl Cancer Inst 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- [8].Montazeri A, Harirchi I, Vahdani M, et al. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): translation and validation study of the Iranian version. Support Care Cancer 1999;7:400–6. [DOI] [PubMed] [Google Scholar]
- [9].Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol 1999;17:1008–19. [DOI] [PubMed] [Google Scholar]
- [10].Bjordal K, de Graeff A, Fayers PM, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. Eur J Cancer 2000;36:1796–807. [DOI] [PubMed] [Google Scholar]
- [11].Sahba S, Talaeepour AR, Hadad P, et al. The efficacy of Iranian made saliva substitute vs. VA-OraLube in improvement of oral-health-related quality of life in radiotherapy-induced xerostomia. J Dent Sch 2009;27:136–45. [Google Scholar]
- [12].Pandey M, Karthikeyan S, Joshi D, et al. Results of a randomized controlled trial of level IIb preserving neck dissection in clinically node-negative squamous carcinoma of the oral cavity. World J Surg Oncol 2018;16:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Landström FJ, Nilsson CO, Reizenstein JA, et al. Electrochemotherapy: possible benefits and limitations to its use in the head and neck region. Acta Otolaryngol 2015;135:90–5. [DOI] [PubMed] [Google Scholar]
- [14].Pierre CS, Dassonville O, Chamorey E, et al. Long-term functional outcomes and quality of life after oncologic surgery and microvascular reconstruction in patients with oral or oropharyngeal cancer. Acta Otolaryngol 2014;134:1086–93. [DOI] [PubMed] [Google Scholar]
- [15].de Melo NB, Bernardino ÍM, de Melo DP, et al. Head and neck cancer, quality of life, and determinant factors: a novel approach using decision tree analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 2018;126:486–93. [DOI] [PubMed] [Google Scholar]
- [16].Moubayed SP, Sampalis JS, Ayad T, et al. Predicting depression and quality of life among long-term head and neck cancer survivors. Otolaryngol Head Neck Surg 2015;152:91–7. [DOI] [PubMed] [Google Scholar]
- [17].Zhu J, Xiao Y, Liu F, et al. Measures of health-related quality of life and socio-cultural aspects in young patients who after mandible primary reconstruction with free fibula flap. World J Surg Oncol 2013;11:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Segna E, Bolzoni AR, Giannì AB, et al. Impact of reconstructive microsurgery on patients with cancer of the head and neck: a prospective study of quality of life, particularly in older patients. Br J Oral Maxillofac Surg 2018;56:830–4. [DOI] [PubMed] [Google Scholar]
- [19].Danielsson D, Munck-Wikland E, Hagel E, et al. Quality of life after microvascular mandibular reconstruction for osteoradionecrosis: a prospective study. Head Neck 2019;41:2225–30. [DOI] [PubMed] [Google Scholar]
- [20].Singer S, Araújo C, Arraras JI, et al. Measuring quality of life in patients with head and neck cancer: update of the EORTC QLQ-H&N Module, Phase III. Head Neck 2015;37:1358–67. [DOI] [PubMed] [Google Scholar]


