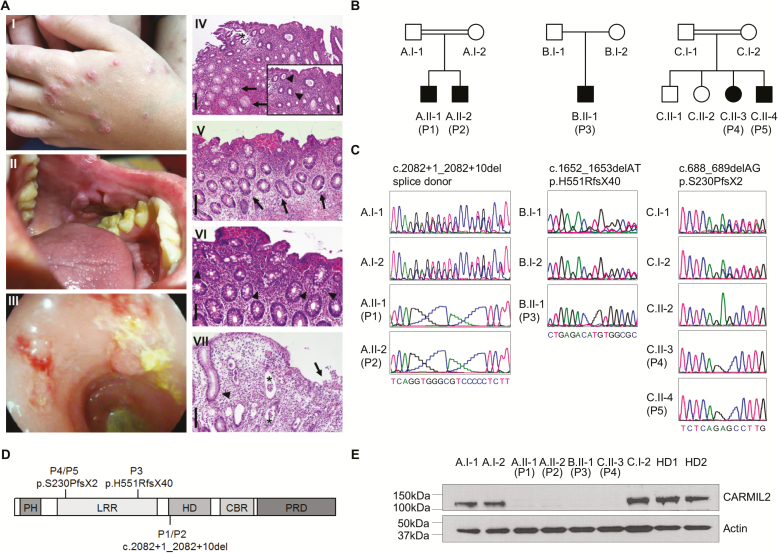
FIGURE 1.
Identification of patients with IBD-like phenotypes and novel loss-of-function mutations in CARMIL2. A, The index patient P2 presented with nodular dermatitis (I), oral aphthous lesions (II), and pancolitis with edematous colonic mucosa with loss of vascularity, ulcerations, fresh bleeding, and pseudopolypoid lesions (III). Histopathological analysis of colonic biopsies from P1, P2, and P3 confirmed active colitis: P1 displayed a moderately active colitis with granulocytic accentuation in the upper third of the mucosa and the surface epithelial layer (inlay; arrowheads) and mucoid metaplasia (arrows) (IV). The asterisk indicates an artificial defect. P2 showed a mildly chronic active colitis with increased epithelial regeneration (arrows, V) and marked increase of eosinophilic granulocytes (arrowheads, VI). P3 displayed a moderately chronic and highly active colitis with mucosal edema and ulceration (arrow), the formation of numerous crypt abscesses (astericks) and regenerative crypt hyperplasia (arrowhead) (VII). Scale bars indicate 200 µm in (IV, V, VII) and 100 µm in (VI, inlay of IV), respectively. B, Pedigrees of 5 patients from 3 unrelated kindred (A–C) with IBD-like symptoms (P1–P5). C, DNA Sanger sequencing confirmed biallelic CARMIL2 mutations. D, Schematic representation of CARMIL2 and localization of the identified mutations. Abbreviations: PH, pleckstrin homology domain; LRR, leucine-rich repeat; HD, homodimerization domain; CBR, capping protein binding region; PRD, proline-rich region. E, Immunoblotting of CARMIL2 protein expression in T lymphoblasts derived from healthy donors (HD), parents, and patients. Actin was used as loading control. Data are representative for 3 experiments.